New malaria vaccine could 'have a major public health impact,' trial suggests

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
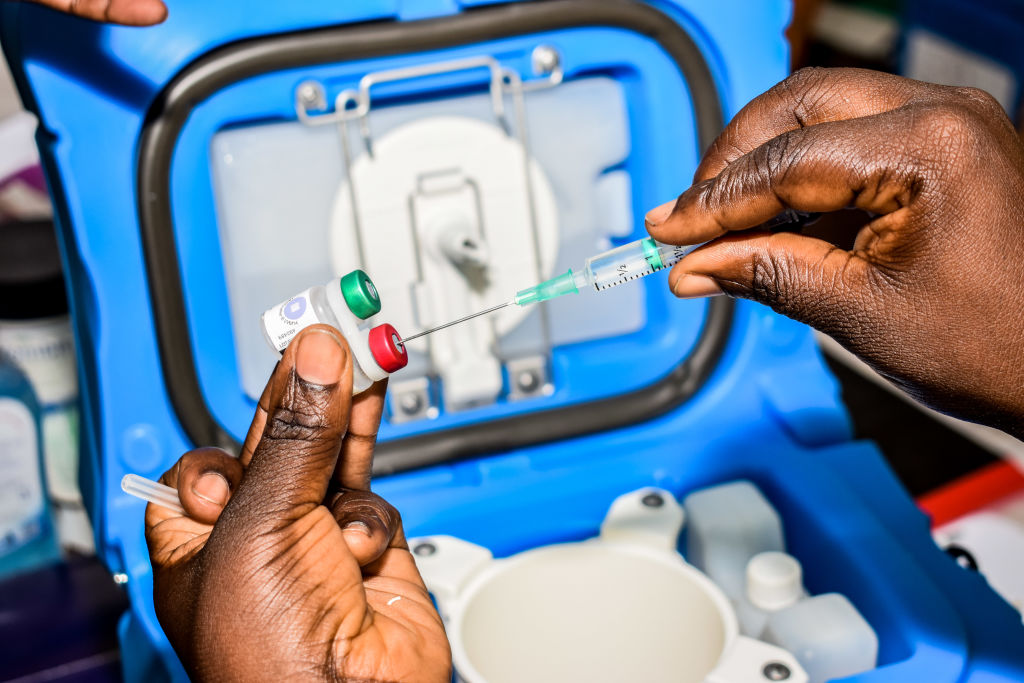
University of Oxford scientists have reportedly developed the first malaria vaccine which, in a trial, surpassed a key goal of greater than 75 percent efficacy.
In a trial consisting of 450 children in Burkina Faso between the ages of five and 17 months, this vaccine candidate was shown to be 77 percent effective against malaria, Bloomberg reports. It also showed a "favorable safety profile and was well-tolerated." The study was published in The Lancet, though it has not yet been peer-reviewed, and the vaccine is set to be studied further in larger clinical trials with 4,800 children in four African countries.
But Bloomberg noted it was the first vaccine against malaria to show greater than 75 percent efficacy, the World Health Organization goal for such a vaccine. According to BBC News, the most effective malaria vaccine to this point showed 55 percent efficacy in trials with African children. There were an estimated 409,000 malaria deaths and 229 million cases worldwide in 2019, the World Health Organization says.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"An effective and safe malaria vaccine would be a hugely significant extra weapon in the armory needed to defeat malaria," Malaria No More U.K.'s Gareth Jenkins said. "Countries freed from the malaria burden will be much better equipped to fight off new disease threats when they inevitably emerge in the future."
Adrian Hill, director of the University of Oxford's Jenner Institute, also told BBC News that the trial suggests this vaccine "has the potential to have a major public health impact."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
