Pfizer and BioNTech say lower dose of COVID-19 vaccine proved safe, effective in kids 5 to 11

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
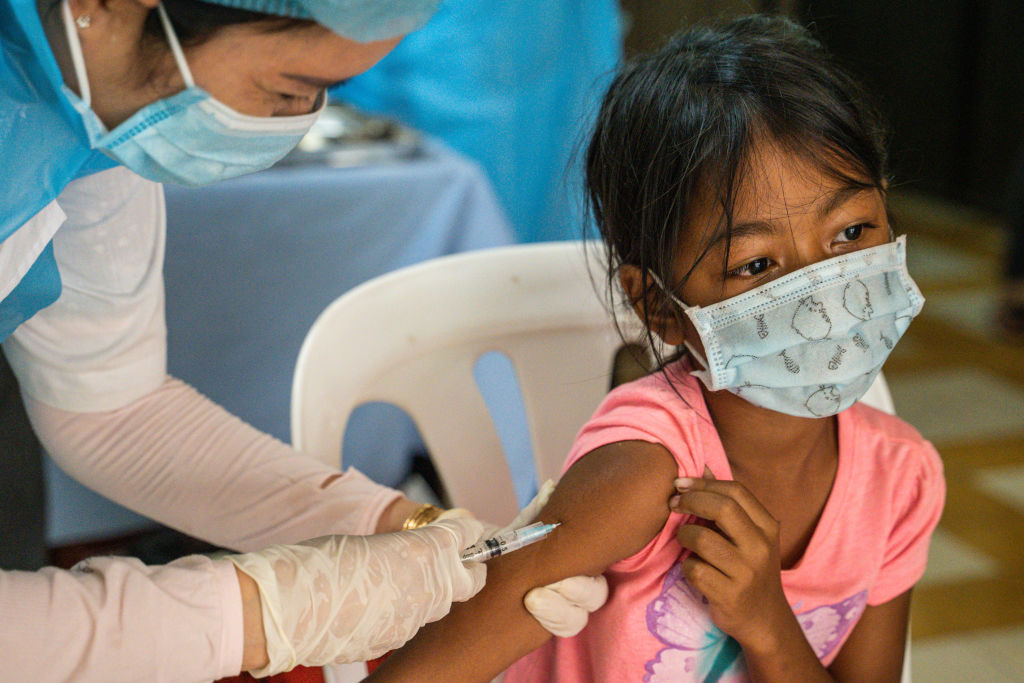
Pfizer and BioNTech said Monday morning that its COVID-19 vaccine was shown to be "safe, well-tolerated, and showed robust neutralizing antibody responses" in children 5 to 11. The 2,268 trial participants in that age group were given two smaller doses of the Pfizer vaccine, and the "results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency," Pfizer CEO Albert Bourla said in a statement.
The Food and Drug Administration has given emergency approval of the vaccine for kids 12 to 15, and full approval to Americans 16 and older.
"We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children," Bourla said. "Since July, pediatric cases of COVID-19 have risen by about 240 percent in the U.S. — underscoring the public health need for vaccination."
Article continues belowThe Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Pfizer is conducting two other ongoing trials, of kids 2 to 5 and children 6 months to 2 years old, and the results for those studies could be in before the end of the year. Nearly 5.3 million children have tested positive for COVID-19 in the U.S., NBC News reports.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
