The FDA has been timid on COVID tests. It should have been bold.

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
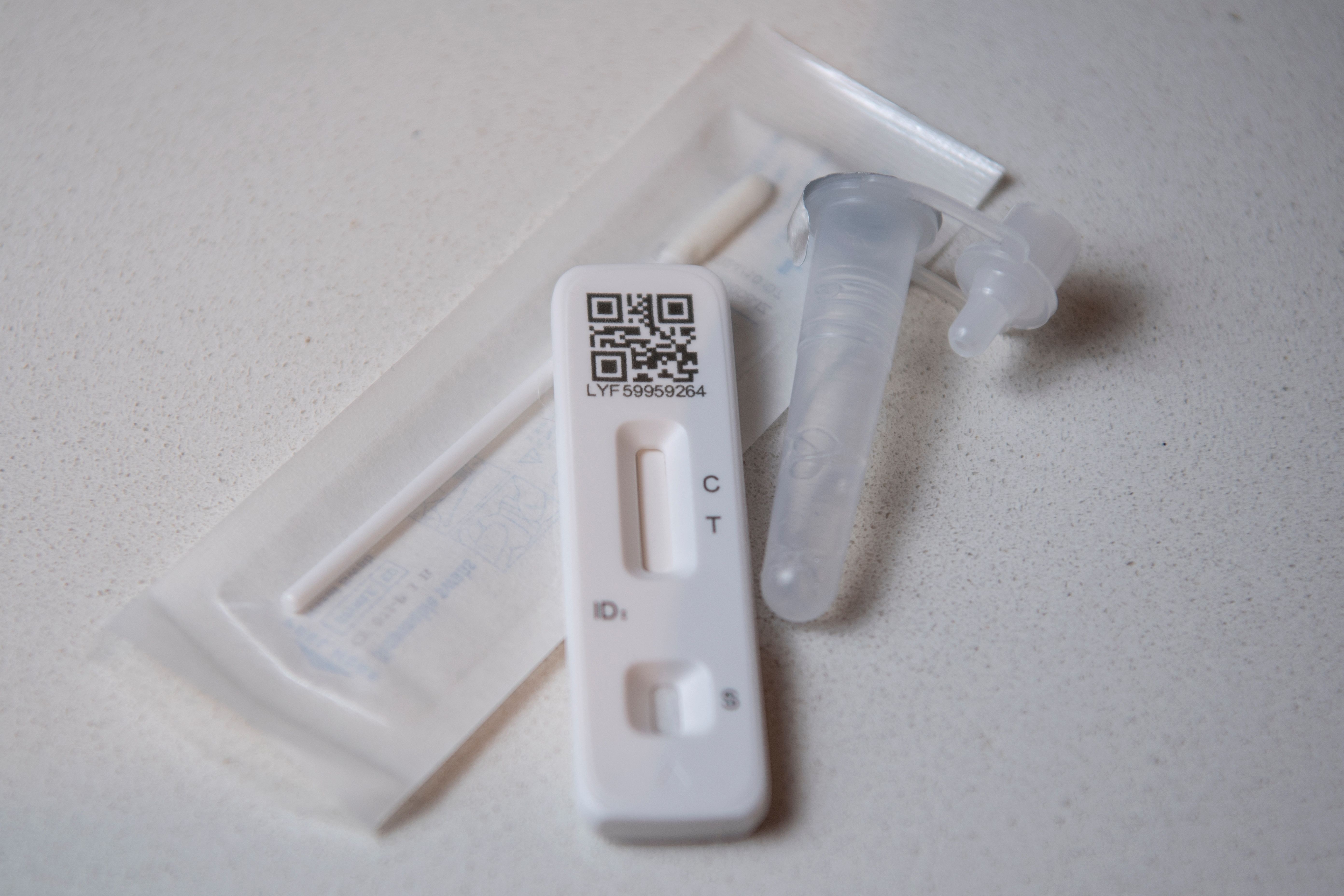
One of the more aggravating aspects of the Omicron surge is that the country needs lots and lots of COVID tests, preferably cheap, but getting them is difficult. It's easier to slow the spread of a virus — or to safely visit family at the holidays — if you know who has the bug and who doesn't. But nearly two years into the pandemic, achieving that simple goal in America is still elusive. (In Europe, testing kits are relatively cheap and easy to obtain.)
The problem seems to be at the FDA. ProPublica reported last month that companies trying to develop rapid COVID tests have encountered an "arbitrary, opaque process" which takes so long to complete that one agency scientist quit in frustration earlier this year. One company was ready to roll out a product in March 2020, right as the pandemic was getting under way, but approval never came.
"You could have antigen tests saving lives since the beginning of the pandemic," said MIT's Irene Bosch, who developed that test. "That's the sad story."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
All of this ought to raise an uncomfortable question for Democrats: Are conservatives right that America's regulatory processes are too much?
Republicans have long been obsessed with deregulation, and in particular they have argued the FDA's approval processes prevent new life-saving treatments from getting to the people who need them. The party has acted accordingly: Then-President Donald Trump signed a federal "right to try" law in 2018 that lets sick patients try experimental drugs. His administration also raised alarms earlier this year when it tried to jam through last-minute directives to reduce the agency's oversight of new drugs and medical devices. The FDA's defenders say its processes try to balance speed and the need to deliver safe, effective care — and present themselves as defenders of the scientific process.
Maybe that's the intent, but the ongoing dearth of cheap and plentiful COVID tests suggests the balance is out of whack. The FDA has been timid where it ought to be bold. And it suggests that Republicans have had a point. Regulations exist to protect Americans from harm, whether that be through illness, injury, or some other cause. In the case of COVID testing, though, the FDA's processes have arguably created more problems than they have prevented.
As the party of Big Government — and as the party currently in charge — Democrats must fix this testing mess. The answer isn't to merely eliminate safety rules and leave Americans at the mercy of the markets, but to make the process more flexible and ready to act in emergencies. It's time to make the system work. Two years is too long to wait for COVID tests.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Joel Mathis is a writer with 30 years of newspaper and online journalism experience. His work also regularly appears in National Geographic and The Kansas City Star. His awards include best online commentary at the Online News Association and (twice) at the City and Regional Magazine Association.
-
 6 of the world’s most accessible destinations
6 of the world’s most accessible destinationsThe Week Recommends Experience all of Berlin, Singapore and Sydney
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 Big-time money squabbles: the conflict over California’s proposed billionaire tax
Big-time money squabbles: the conflict over California’s proposed billionaire taxTalking Points Californians worth more than $1.1 billion would pay a one-time 5% tax
-
 Did Alex Pretti’s killing open a GOP rift on guns?
Did Alex Pretti’s killing open a GOP rift on guns?Talking Points Second Amendment groups push back on the White House narrative
-
 Washington grapples with ICE’s growing footprint — and future
Washington grapples with ICE’s growing footprint — and futureTALKING POINTS The deadly provocations of federal officers in Minnesota have put ICE back in the national spotlight
-
 Trump’s Greenland ambitions push NATO to the edge
Trump’s Greenland ambitions push NATO to the edgeTalking Points The military alliance is facing its worst-ever crisis
-
 Why is Trump threatening defense firms?
Why is Trump threatening defense firms?Talking Points CEO pay and stock buybacks will be restricted
-
 The billionaires’ wealth tax: a catastrophe for California?
The billionaires’ wealth tax: a catastrophe for California?Talking Point Peter Thiel and Larry Page preparing to change state residency
-
 Trump considers giving Ukraine a security guarantee
Trump considers giving Ukraine a security guaranteeTalking Points Zelenskyy says it is a requirement for peace. Will Putin go along?
-
 Bari Weiss’ ‘60 Minutes’ scandal is about more than one report
Bari Weiss’ ‘60 Minutes’ scandal is about more than one reportIN THE SPOTLIGHT By blocking an approved segment on a controversial prison holding US deportees in El Salvador, the editor-in-chief of CBS News has become the main story
