Pfizer asks FDA to authorize its COVID vaccine for use in kids under 5

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
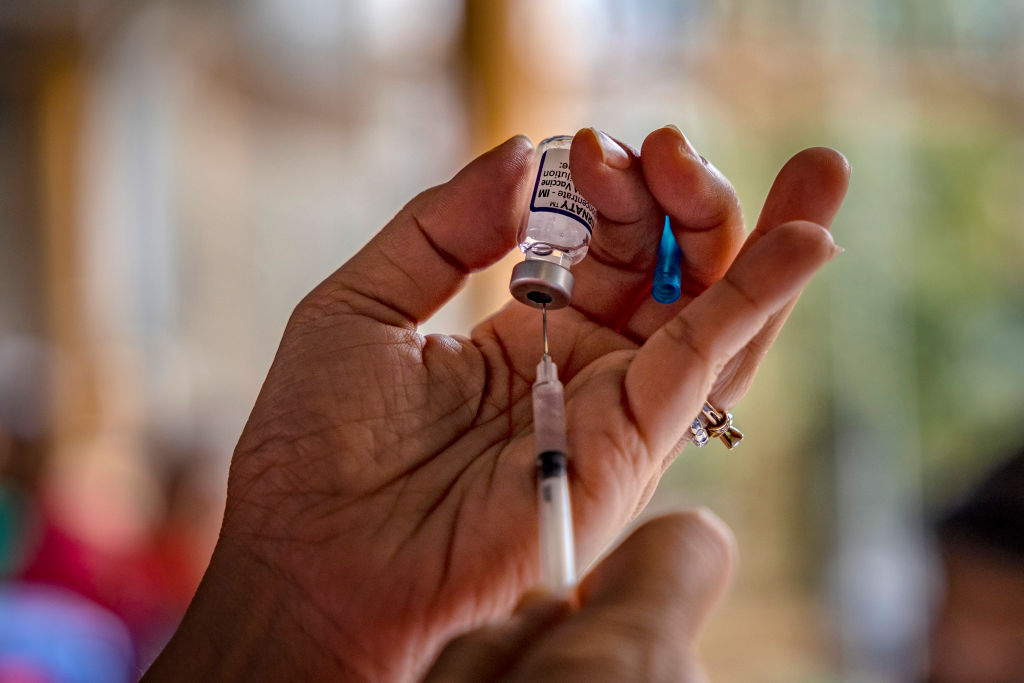
Pfizer and BioNTech have requested emergency authorization from the Food and Drug Administration to use their COVID-19 vaccine in children ages 6 months to 5 years, the companies announced Tuesday, following Monday reports.
If authorized, Pfizer's would be the first COVID vaccine available for kids under five, CNN reports. The two companies are also "continuing to test a three-dose version of the vaccine in this younger age group."
The youngest kids will receive the smallest dose: 3 micrograms a shot.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Federal regulators encouraged Pfizer to submit data for authorization for the two-dose vaccine, rather than wait on data for three doses, which could have potentially delayed the process until March, CNN notes. The two-dose regimen notably produced "disappointing results in some children during testing," leading researchers to believe another dose may eventually be required for full protection, reports The Wall Street Journal.
"If the goal of the vaccine is to get baseline immunity in the kids — to prevent really bad outcomes and you're really not using the vaccine as a tool to prevent infection in the first place — two doses could do that," former FDA commissioner and current Pfizer board member Dr. Scott Gottlieb said Sunday, per CNN. "I think that may be why federal health officials are rethinking this."
On that note, the FDA reportedly encouraged Pfizer to forge ahead with authorization of the two-shot regimen so kids would be eligible for a third dose later, should it prove safe and effective, the Journal adds. There were no serious safety concerns during testing, the companies said.
There are over 19 million Americans under 5 years old, The New York Times reports.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brigid Kennedy worked at The Week from 2021 to 2023 as a staff writer, junior editor and then story editor, with an interest in U.S. politics, the economy and the music industry.
-
 Political cartoons for February 12
Political cartoons for February 12Cartoons Thursday's political cartoons include a Pam Bondi performance, Ghislaine Maxwell on tour, and ICE detention facilities
-
 Arcadia: Tom Stoppard’s ‘masterpiece’ makes a ‘triumphant’ return
Arcadia: Tom Stoppard’s ‘masterpiece’ makes a ‘triumphant’ returnThe Week Recommends Carrie Cracknell’s revival at the Old Vic ‘grips like a thriller’
-
 My Father’s Shadow: a ‘magically nimble’ film
My Father’s Shadow: a ‘magically nimble’ filmThe Week Recommends Akinola Davies Jr’s touching and ‘tender’ tale of two brothers in 1990s Nigeria
-
 Blue Origin launches Mars probes in NASA debut
Blue Origin launches Mars probes in NASA debutSpeed Read The New Glenn rocket is carrying small twin spacecraft toward Mars as part of NASA’s Escapade mission
-
 Dinosaurs were thriving before asteroid, study finds
Dinosaurs were thriving before asteroid, study findsSpeed Read The dinosaurs would not have gone extinct if not for the asteroid
-
 SpaceX breaks Starship losing streak in 10th test
SpaceX breaks Starship losing streak in 10th testspeed read The Starship rocket's test flight was largely successful, deploying eight dummy satellites during its hour in space
-
 Rabbits with 'horns' sighted across Colorado
Rabbits with 'horns' sighted across Coloradospeed read These creatures are infected with the 'mostly harmless' Shope papilloma virus
-
 Lithium shows promise in Alzheimer's study
Lithium shows promise in Alzheimer's studySpeed Read Potential new treatments could use small amounts of the common metal
-
 Scientists discover cause of massive sea star die-off
Scientists discover cause of massive sea star die-offSpeed Read A bacteria related to cholera has been found responsible for the deaths of more than 5 billion sea stars
-
 'Thriving' ecosystem found 30,000 feet undersea
'Thriving' ecosystem found 30,000 feet underseaSpeed Read Researchers discovered communities of creatures living in frigid, pitch-black waters under high pressure
-
 New York plans first nuclear plant in 36 years
New York plans first nuclear plant in 36 yearsSpeed Read The plant, to be constructed somewhere in upstate New York, will produce enough energy to power a million homes
