Solving COVID: February 24, 2021
Pfizer and Moderna to ramp up vaccine shipments, FDA says J&J vaccine is safe and effective, and more
- 1. Pfizer and Moderna plan to at least double vaccine shipments by mid-March
- 2. FDA confirms Johnson & Johnson vaccine is safe and effective
- 3. COVID-19 survivors may be protected with just 1 vaccine dose, early studies suggest
- 4. Pfizer-BioNTech COVID-19 vaccine curbs transmission, Israeli analysis suggests
- 5. Pfizer vaccine can be stored in regular freezers
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
1. Pfizer and Moderna plan to at least double vaccine shipments by mid-March
Pfizer/BioNTech and Moderna pledged Tuesday to boost their current distribution of COVID-19 vaccines. As it stands, Pfizer and Moderna are distributing 4-5 million vaccine doses each week. Pfizer plans to up that to 13 million doses weekly by mid-March, and Moderna is working to distribute 40 million doses per month, the companies told the House Energy and Commerce Committee during a Tuesday hearing. Moderna plans to ship at least 100 million doses by the end of May. The increased production promises come amid a sluggish coronavirus vaccine rollout; President Biden has set the U.S. on a goal of distributing 1.5 million doses each day. Both companies are currently testing booster shots that may work better against more transmissible COVID-19 variants, and Moderna is testing its vaccine's efficacy on older children.
2. FDA confirms Johnson & Johnson vaccine is safe and effective
A Food and Drug Administration review confirmed Wednesday that Johnson & Johnson's COVID-19 vaccine, which only requires a single shot, is safe and effective, meaning it could be authorized for emergency approval by this weekend. The FDA review showed the vaccine in a large clinical trial was 66 percent effective at preventing moderate to severe COVID-19, though it was 85 percent effective at preventing severe illness. The vaccine in the clinical trial also completely protected against COVID-19 hospitalizations and deaths 28 days after vaccination. A committee is set to meet Friday to consider whether the FDA should authorize the Johnson & Johnson vaccine, which can be stored for three months in a refrigerator, for emergency use.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The Washington Post The Associated Press
3. COVID-19 survivors may be protected with just 1 vaccine dose, early studies suggest
As COVID-19 vaccine doses remain in short supply, new preliminary studies suggest only giving one dose to people who've recovered from the disease is enough to protect them from reinfection. Past studies have shown COVID-19 survivors are left with antibodies that help protect them from reinfection for at least a few months, depending on how severe their infection was. But multiple not-yet-peer-reviewed studies suggest they need another boost from a vaccine, and when they get the first dose, they experience far higher levels of protection than people who get the first dose but weren't previously infected. That dose is especially important when it comes to fighting off the B.1.351 variant of the virus, as studies show COVID-19 survivors' natural immunity may not be as effective as a vaccine against the strain.
The Wall Street Journal The New York Times
4. Pfizer-BioNTech COVID-19 vaccine curbs transmission, Israeli analysis suggests
Data from Israel that are awaiting publication suggest the Pfizer-BioNTech COVID-19 vaccine is not only effective at preventing symptomatic infection, but can also stop transmission of the virus in the first place, the Financial Times and Bloomberg reported Sunday. The results were compiled by the health ministry in Israel, the country with the world's highest vaccination rate, in the three weeks leading up to Feb. 6. By the end of that period, more than 27 percent of the over-15 Israeli population had been fully vaccinated, and the preliminary real-world observation analysis found the vaccine was 89 percent effective at preventing infection of any kind. As is often the case with studies during the coronavirus pandemic, the findings require more scrutiny, but they are in line with those of a similar analysis conducted by the Mayo Clinic, which found the Pfizer and Moderna vaccines were around 80 percent effective at preventing infection.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
5. Pfizer vaccine can be stored in regular freezers
The Pfizer/BioNTech COVID-19 vaccine faced severe limitations for the first few months of its distribution because it had to be stored in an ultracold freezer — something everyday clinics and impromptu vaccine centers don't have. But the two companies revised that guidance on Friday, saying the vaccines only need to be stored in a regular freezer. They're now looking to officially change the vaccine's storage guidance with the Food and Drug Administration. Researchers on Thursday also found Pfizer's vaccine grants 92.6 percent immunity against the virus after just one dose, suggesting the U.S. should try to get first shots to more people before moving on with the second dose. A peer-reviewed study from Israel out Friday meanwhile found it was 85 percent effective in preventing infection 15 to 28 days after it's administered.
-
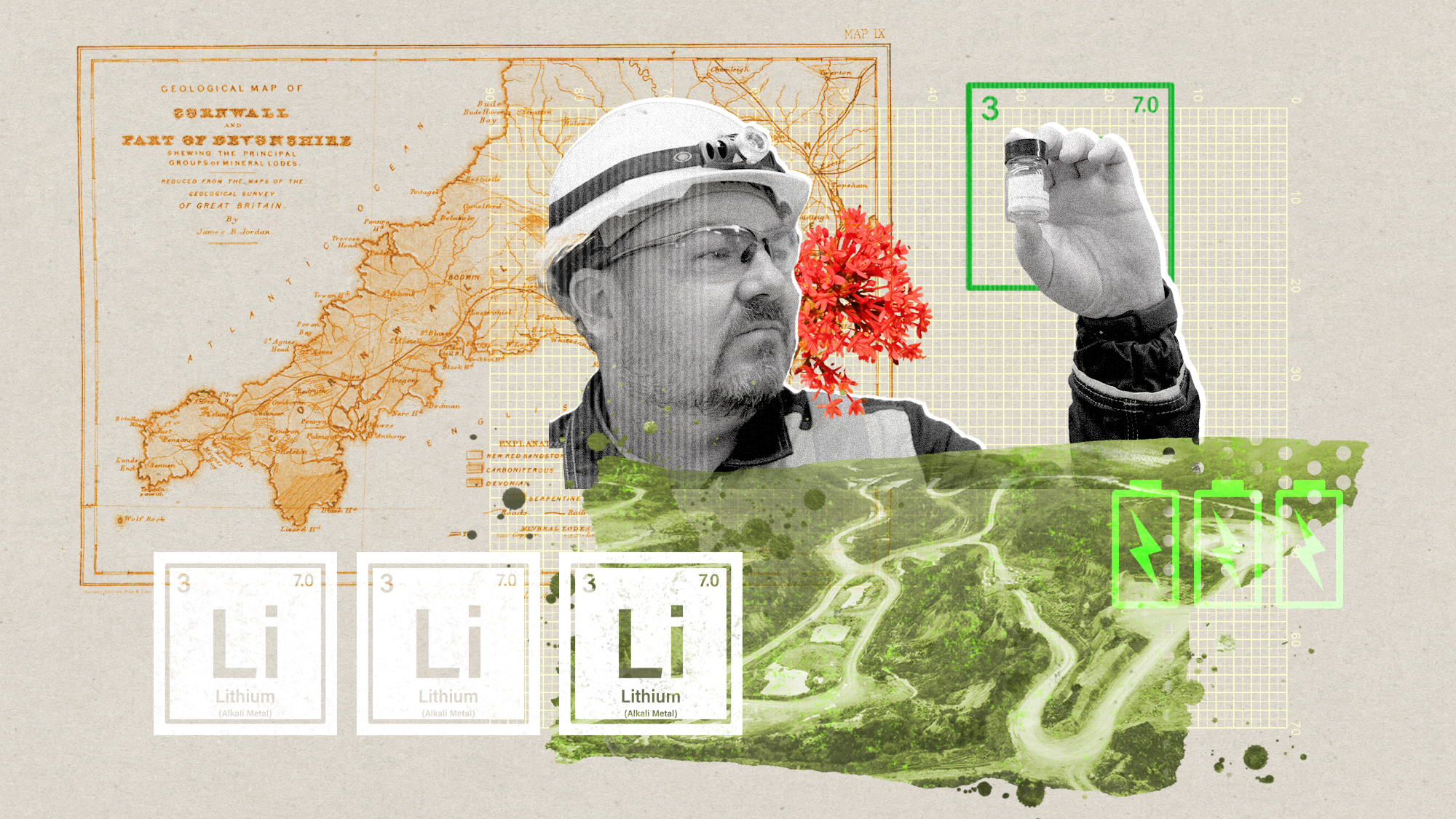 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 Epstein files topple law CEO, roil UK government
Epstein files topple law CEO, roil UK governmentSpeed Read Peter Mandelson, Britain’s former ambassador to the US, is caught up in the scandal
-
 Iran and US prepare to meet after skirmishes
Iran and US prepare to meet after skirmishesSpeed Read The incident comes amid heightened tensions in the Middle East
-
 Israel retrieves final hostage’s body from Gaza
Israel retrieves final hostage’s body from GazaSpeed Read The 24-year-old police officer was killed during the initial Hamas attack
-
 China’s Xi targets top general in growing purge
China’s Xi targets top general in growing purgeSpeed Read Zhang Youxia is being investigated over ‘grave violations’ of the law
-
 Panama and Canada are negotiating over a crucial copper mine
Panama and Canada are negotiating over a crucial copper mineIn the Spotlight Panama is set to make a final decision on the mine this summer
-
 Why Greenland’s natural resources are nearly impossible to mine
Why Greenland’s natural resources are nearly impossible to mineThe Explainer The country’s natural landscape makes the task extremely difficult
-
 Iran cuts internet as protests escalate
Iran cuts internet as protests escalateSpeed Reada Government buildings across the country have been set on fire
-
 US nabs ‘shadow’ tanker claimed by Russia
US nabs ‘shadow’ tanker claimed by RussiaSpeed Read The ship was one of two vessels seized by the US military