FDA approves COVID-19 booster shot for transplant recipients, other immunocompromised Americans

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
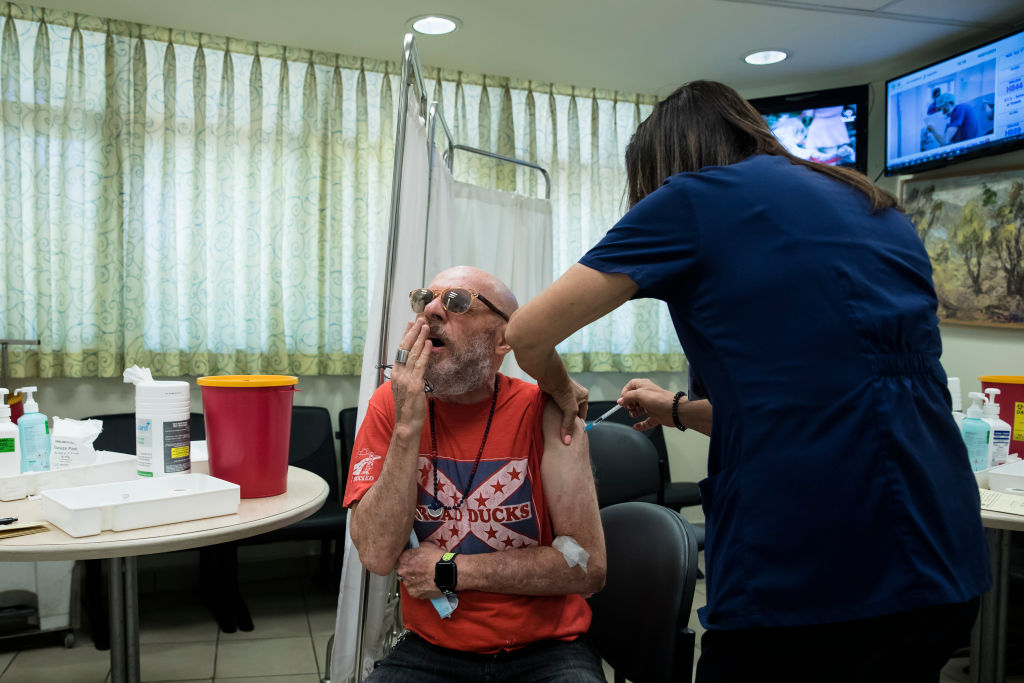
The Food and Drug Administration on Thursday night gave formal approval for severely immunocompromised Americans to get a third shot of the Pfizer-BioNTech and Moderna COVID-19 vaccines. Studies have shown that transplant recipients and others with seriously weakened immune systems don't get the same level of protection against the coronavirus from the first two doses, but many mount a stronger immune response after a third dose.
"Today's action allows doctors to boost immunity in certain immunocompromised individuals who need extra protection from COVID-19," said Dr. Janet Woodcock, the FDA's acting commissioner. Less than 3 percent of the U.S. population will be approved for the booster shot, and the FDA's statement did not mention immunocompromised people who received the one-dose Johnson & Johnson vaccine. And it isn't clear which high-risk groups would benefit from a booster shot.
"This is all going to be very personalized," said Dr. Dorry Segev, a transplant surgeon at Johns Hopkins University who is conducting a National Institutes of Health study on booster shots for organ transplant recipients. But it's clear the risks are greater for the immunocompromised. One recent study led by Sergev found that vaccinated transplant recipients are 82 times more likely to get a breakthrough COVID-19 infection and 485 times more likely to be hospitalized or die from the coronavirus.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
U.S. health officials are closely monitoring how long the vaccines protect people without suppressed immune systems, with an eye to whether booster shots will eventually be necessary for the general population. Everyone will likely need a booster shot at some point, White House coronavirus adviser Dr. Anthony Fauci said at a briefing Thursday, but "apart from the immunocompromised, we do not believe that others, elderly or non-elderly, need a vaccine at this moment."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’
Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’Feature An inconvenient love torments a would-be couple, a gonzo time traveler seeks to save humanity from AI, and a father’s desperate search goes deeply sideways
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 Regent Hong Kong: a tranquil haven with a prime waterfront spot
Regent Hong Kong: a tranquil haven with a prime waterfront spotThe Week Recommends The trendy hotel recently underwent an extensive two-year revamp
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
