Tianeptine: why lawmakers fear a new kind of opioid
The drug is sold over the counter. And is highly addictive.

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. is suffering from an opioid problem, and now a dangerous drug is being sold in the country's gas stations and convenience stores. This especially accessible drug, tianeptine, is marketed as a dietary supplement but, in a manner similar to opioids, is causing addiction and overdose deaths across the country. Lawmakers want the government to take stronger measures against the distribution of tianeptine.
What is tianeptine?
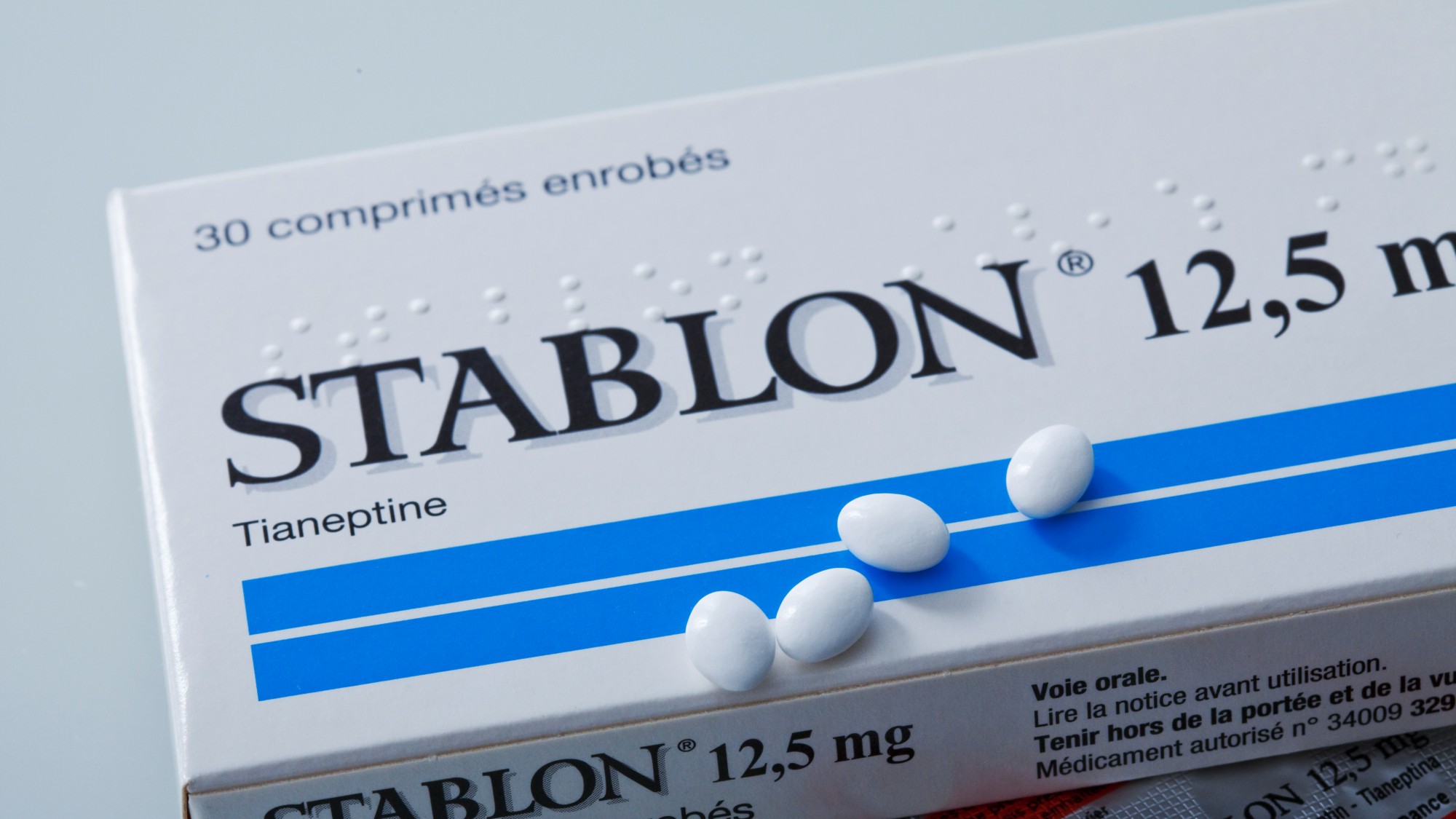
Tianeptine, also called "gas station heroin," is an unregulated antidepressant "promoted by retailers as a mood booster and focus aid," The New York Times explained. The drug is not approved for use in the U.S. and "although other countries have approved tianeptine to treat depression and anxiety, some have restricted how tianeptine is prescribed or dispensed, or revised the drug label to warn of possible addiction," according to the U.S. Food and Drug Administration (FDA). Many have used the drug as a way to get high, as a dietary supplement or to self-medicate for depression and anxiety. Tianeptine has been sold under many brand names, including Stablon, Tatinol and Coaxil, and as an ingredient in products marketed as dietary supplements such as Tianaa, Neptune's Fix, Zaza and Pegasusin. The drug is available across the U.S. in gas stations and convenience stores and can also be purchased online.
Researchers have found that using tianeptine has similar effects to using opioids. "We have people who are able to get a substance that's not well regulated, that has abuse potential and that, in high doses, can cause similar effects to opioids, leading to really harmful outcomes," Kaitlyn Brown, clinical managing director of America's Poison Centers, told the Times. There are also no quality and purity standards that tianeptine manufacturers abide by. Sometimes naloxone, a drug created to reverse opioid overdoses, can help with tianeptine overdoses, but it doesn't show consistent effectiveness. There were 391 cases of tianeptine exposure reported to America's Poison Centers in 2023, compared to only four in 2013.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Because of the lack of regulation, "consumers are usually taking a gamble when they buy tianeptine products because they can't know for sure how much of the drug they're getting," according to Popular Science. This can lead to unwanted overdoses and other side effects associated with opioid use and include "lethargy, elevated blood pressure and heart rate, agitation, abdominal pain, tremors and hallucinations." The source added, "Those who regularly misuse the pills may exhibit withdrawal symptoms such as stomachaches and anxiety if they try to quit."
What actions are being taken?
While the FDA has warned about tianeptine, "more action on tianeptine use is needed to ensure the health and well-being of the American people," according to a letter written by five House representatives to the FDA commissioner. Currently, Alabama, Florida, Georgia, Indiana, Kentucky, Michigan, Mississippi, Ohio and Tennessee have banned the drug.
"There are now at least a dozen different products that are foreign drugs being openly marketed as dietary supplements right under the FDA's eyes, without them being able to stop the sales," Dr. Pieter Cohen, an associate professor at Harvard Medical School, told The New York Times. The FDA advised on its website that "consumers should avoid all products containing tianeptine, including those claiming to treat an ailment or disorder." However, the agency hasn't taken any further action to regulate the drug. A representative for the FDA wrote to Popular Science, stating that "the FDA generally cannot confirm or deny the existence of any possible product application," and "a drug sponsor would need to submit an application to the agency for review," in order for it to be approved or denied for medical use. In the meantime, individual states alone are responsible for placing regulations on the drug.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Devika Rao has worked as a staff writer at The Week since 2022, covering science, the environment, climate and business. She previously worked as a policy associate for a nonprofit organization advocating for environmental action from a business perspective.
-
 Local elections 2026: where are they and who is expected to win?
Local elections 2026: where are they and who is expected to win?The Explainer Labour is braced for heavy losses and U-turn on postponing some council elections hasn’t helped the party’s prospects
-
 6 of the world’s most accessible destinations
6 of the world’s most accessible destinationsThe Week Recommends Experience all of Berlin, Singapore and Sydney
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Stopping GLP-1s raises complicated questions for pregnancy
Stopping GLP-1s raises complicated questions for pregnancyThe Explainer Stopping the medication could be risky during pregnancy, but there is more to the story to be uncovered
-
 Nitazene is quietly increasing opioid deaths
Nitazene is quietly increasing opioid deathsThe explainer The drug is usually consumed accidentally
-
 Tips for surviving loneliness during the holiday season — with or without people
Tips for surviving loneliness during the holiday season — with or without peoplethe week recommends Solitude is different from loneliness
-
 More women are using more testosterone despite limited research
More women are using more testosterone despite limited researchThe explainer There is no FDA-approved testosterone product for women
-
 Climate change is getting under our skin
Climate change is getting under our skinUnder the radar Skin conditions are worsening because of warming temperatures
-
 Can TrumpRx really lower drug prices?
Can TrumpRx really lower drug prices?Today’s Big Question Pfizer’s deal with Trump sent drugmaker stocks higher
-
 The UK’s opioid crisis: why the stats don’t add up
The UK’s opioid crisis: why the stats don’t add upThe Explainer A new report has revealed that the UK’s total of opioid-related deaths could be much greater than official figures show
