Pfizer launches human trials for coronavirus vaccine, aims for emergency use in September


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Pfizer, in partnership with German pharmaceutical company BioNTech, became the latest company to launch human trials for a potential coronavirus vaccine Monday.
If all goes well, the companies hope their COVID-19 vaccine could be ready for emergency use in September, in time for what many predict will be a period of rejuvenated viral transmission (if it subsides at all in the U.S. between now and then). The normal caveats apply here — the quest for a vaccine is moving along at a pace never before seen, which means that some essential steps may be purposefully skipped in the process, and many experts believe that getting a vaccine in even 18 months, let alone later this year, is overly optimistic.
Pfizer and some other companies are using a genetic material known as messenger RNA to develop their vaccine that could possibly train cells to create a protein the coronavirus latches onto without making a person sick. With the protein in the body, a person's immune system could then reportedly produce antibodies ready to fight off a future infection. The good news is that this technology is reportedly more stable than traditional vaccines which use weakened virus strains, and it's faster to produce, hence Pfizer's ambitious timeline. The catch is that no RNA messenger vaccine has ever reached the market before.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
In other coronavirus development news, the United Arab Emirates found that a "minimally invasive" treatment in which stem cells are turned into a mist to be inhaled has resulted in "favorable" outcomes for COVID-19 patients and could hit the market in three months if trials keep going well. Israel, meanwhile, said it has isolated a key coronavirus antibody that could possibly "neutralize" the virus in a patient's body.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
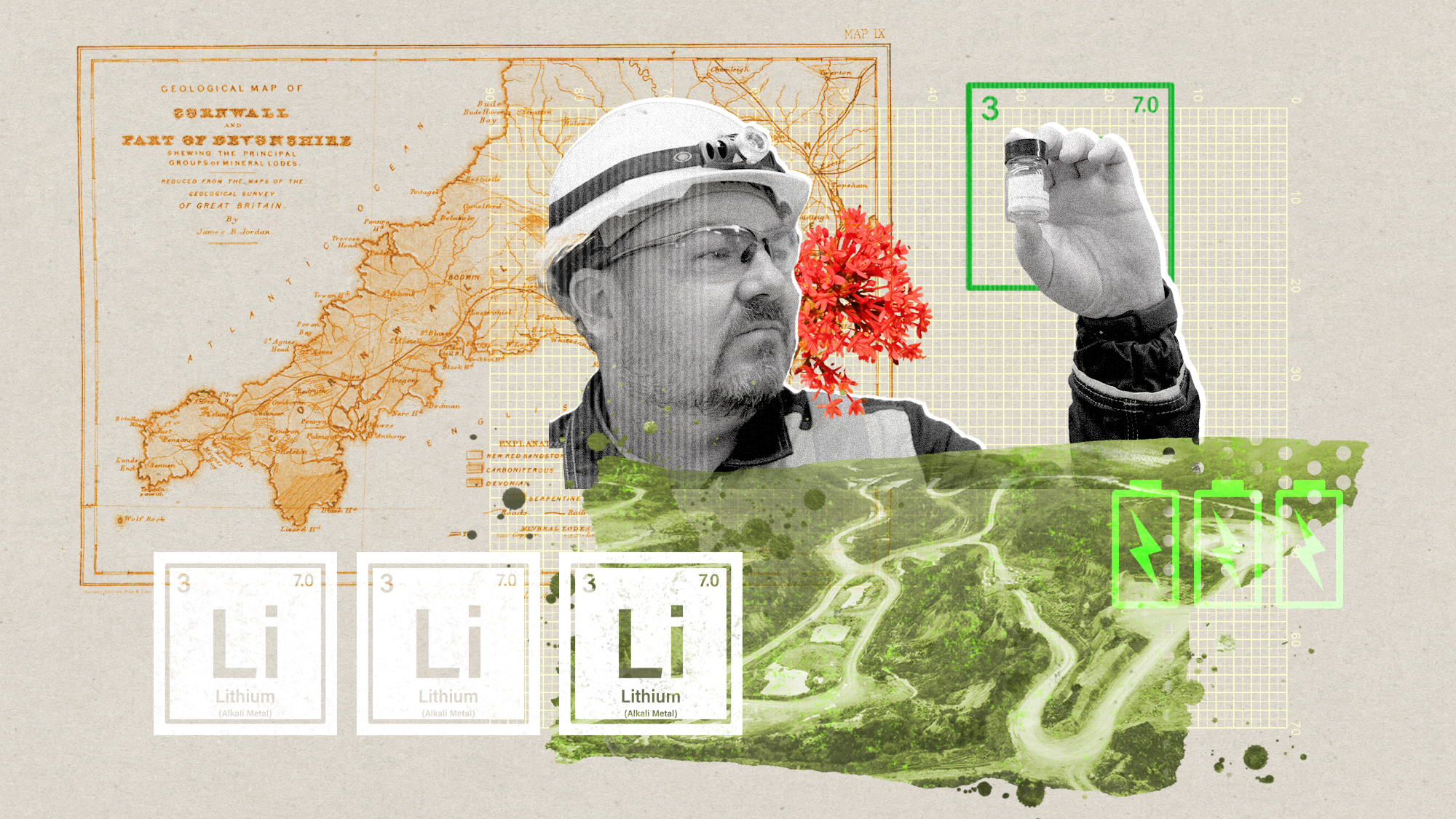 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
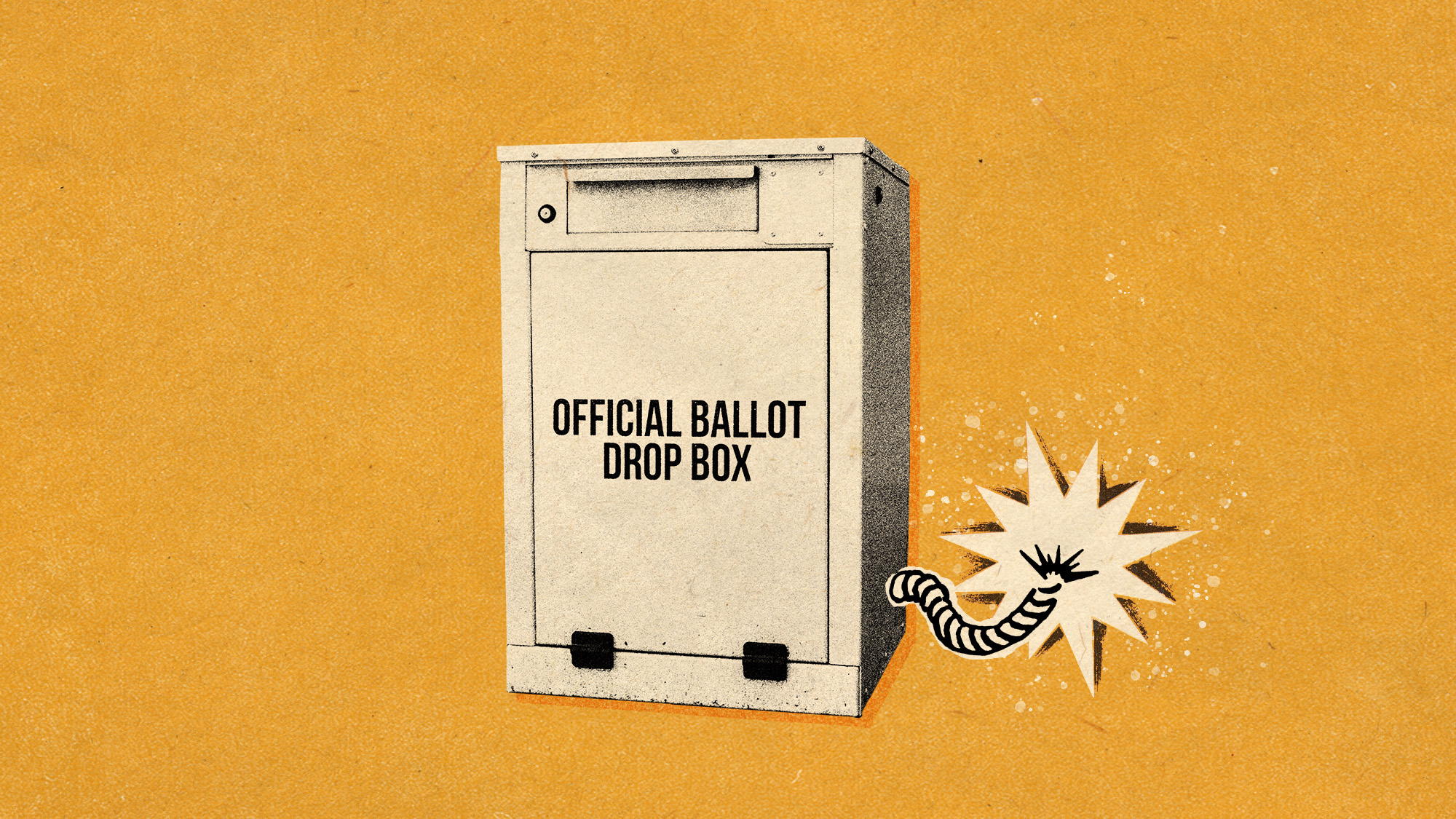 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
