Coronavirus may be safely treated with recovered patients' plasma, study shows

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
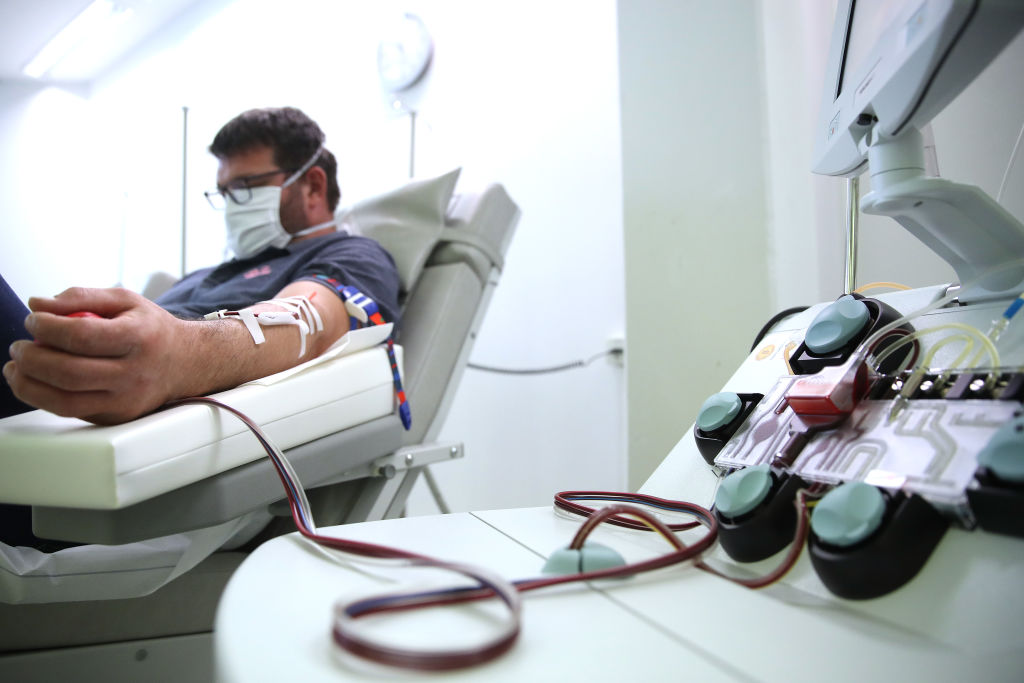
A not-yet-peer-reviewed study seems to pave the way for treating coronavirus with plasma from recovered patients.
With no treatments approved for treating COVID-19 yet, doctors around the country have been trying a variety of drugs and therapies in hopes of finding something promising and further studying it. One of those experimental treatments, known as convalescent plasma transfusions, appears to be safe and could be a worthwhile treatment after it's studied more, an unreviewed study published Thursday on a public server called Medrxiv suggests.
Researchers at the Mayo Clinic, Michigan State University, and Johns Hopkins University, looked at 5,000 coronavirus patients around the country who received convalescent plasma transfusions from recovered patients, The Wall Street Journal notes via the study. Under one percent of the treated patients saw "serious adverse effects" after the transfusions, and there was a 14.9 percent mortality rate seven days after transfusions. Two-thirds of these patients were critically ill to begin with, so "the mortality rate does not appear excessive," the study said.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
While this study doesn't determine whether convalescent transfusions effectively treat coronavirus, it does suggest they are safe for wider research. This study will need to be peer reviewed and replicated in official medical journals before it can be considered an approved way of treating COVID-19. The Food and Drug Administration did approve the "compassionate use" of convalescent plasma treatments in emergency situations as part of its attempts to find an effective therapy.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Kathryn is a graduate of Syracuse University, with degrees in magazine journalism and information technology, along with hours to earn another degree after working at SU's independent paper The Daily Orange. She's currently recovering from a horse addiction while living in New York City, and likes to share her extremely dry sense of humor on Twitter.
-
 Witkoff and Kushner tackle Ukraine, Iran in Geneva
Witkoff and Kushner tackle Ukraine, Iran in GenevaSpeed Read Steve Witkoff and Jared Kushner held negotiations aimed at securing a nuclear deal with Iran and an end to Russia’s war in Ukraine
-
 What to expect financially before getting a pet
What to expect financially before getting a petthe explainer Be responsible for both your furry friend and your wallet
-
 Pentagon spokesperson forced out as DHS’s resigns
Pentagon spokesperson forced out as DHS’s resignsSpeed Read Senior military adviser Col. David Butler was fired by Pete Hegseth and Homeland Security spokesperson Tricia McLaughlin is resigning
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
