Pfizer's coronavirus vaccine candidate is 'viable' — but there are some side effects


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The first clinical data on the vaccine candidate produced by pharmaceutical giant Pfizer in partnership with German biotech firm BioNTech showed some positive results, although there were side effects, Stat News reports.
The study randomly assigned 45 patients one of three doses of the vaccine — which relies on experimental messenger-RNA technology — or a placebo. The bad news is half of the patients who received the highest dose of the vaccine developed fevers, so they weren't given a second injection, but those who received the two lower doses did receive a second dose. After the follow-up shot, more than 50 percent of the volunteers reported some kind of adverse effect, including fevers and sleep disturbances. That's troublesome, but none of the side effects were considered life-threatening or resulted in hospitalization or disability.
Now, for the good news. The vaccine generated neutralizing antibodies that prevent the coronavirus from functioning, and the levels of those neutralizing antibodies were 1.8 to 2.8-times the levels found in recovered COVID-19 patients.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
No one knows if antibodies lead to immunity, and Pfizer, like every manufacturer with a potential vaccine candidate, will have to conduct larger studies to figure that out. Despite the uncertainty and the side effects, the initial findings represent a promising first step. "We still have a ways to go and we're testing other candidates, as well," said Philip Dormitzer, the chief scientific officer for viral vaccines at Pfizer. "However, what we can say at this point is there is a viable vaccine candidate based on immunogenicity and early tolerability safety data." Read more at Stat News.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
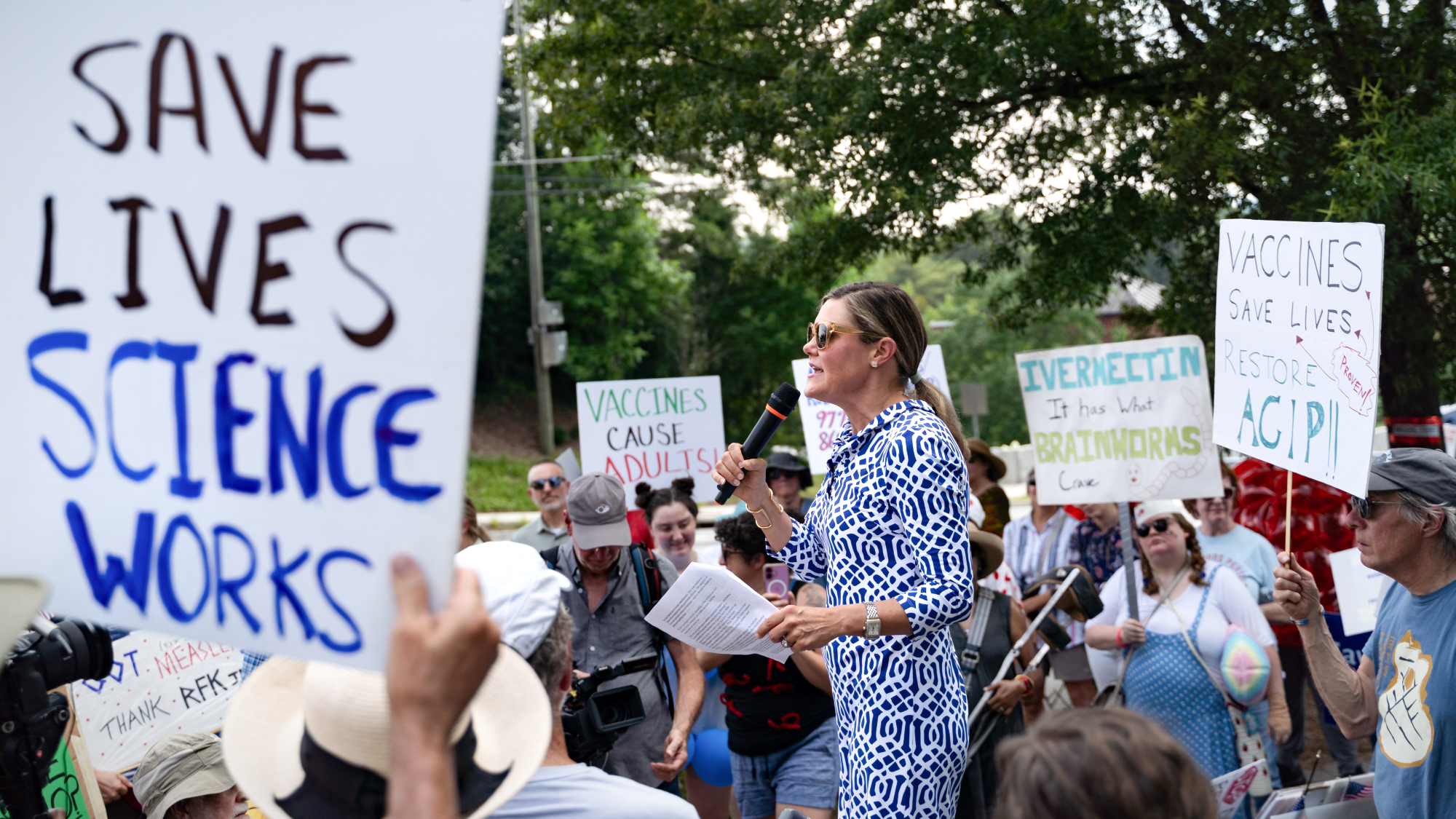 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
