Coronavirus vaccine testers see fertile grounds in Texas, Florida, and Arizona


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. government is planning to fund three 30,000-subject phase 3 COVID-19 vaccine trials starting this month, and Pfizer is recruiting for its own similarly large vaccine trial. "Quickly lining up all the subjects for so many studies at the same time poses several challenges," The Wall Street Journal reports. "We not only have to find the number of volunteers," National Institutes of Health Director Francis Collins explained, "but they need to be in an area where the virus is currently spreading, otherwise you learn nothing about the effectiveness of the vaccine."
The volunteers also need to be healthy and include sufficient high-risk groups that regulators can be sure the vaccines will be safe and effective in the broader population. That means COVID-19 hotspots are seen as fertile ground for recruiting, the Journal reports:
Among the areas being targeted in the U.S. and outside the country, industry officials say, are places where people generally aren't following preventive measures like social distancing or wearing masks. Some testing sites for Pfizer's vaccine trial will be in states that have seen recent increases in infections, such as Florida, Arizona, and Texas, Pfizer Chief Executive Albert Bourla said during a recent online event hosted by the Milken Institute. [The Wall Street Journal]
The need to quickly procure volunteers that meet these criteria has effectively created competition between vaccine trials. Vaccine developers and recruitment organizations are using novel techniques to find such volunteers, including working with churches and community groups, trawling testing centers and pharmacies, using algorithms, and asking employees to reach out to friends and family.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
There are about 150 COVID-19 vaccines under development, and the three large late-stage trials being funded by the U.S. government this summer are for Moderna's vaccine candidate, the U.S. trial of a drug developed by Oxford University and AstraZeneca that's already being tested in Britain, and Johnson & Johnson's vaccine effort.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
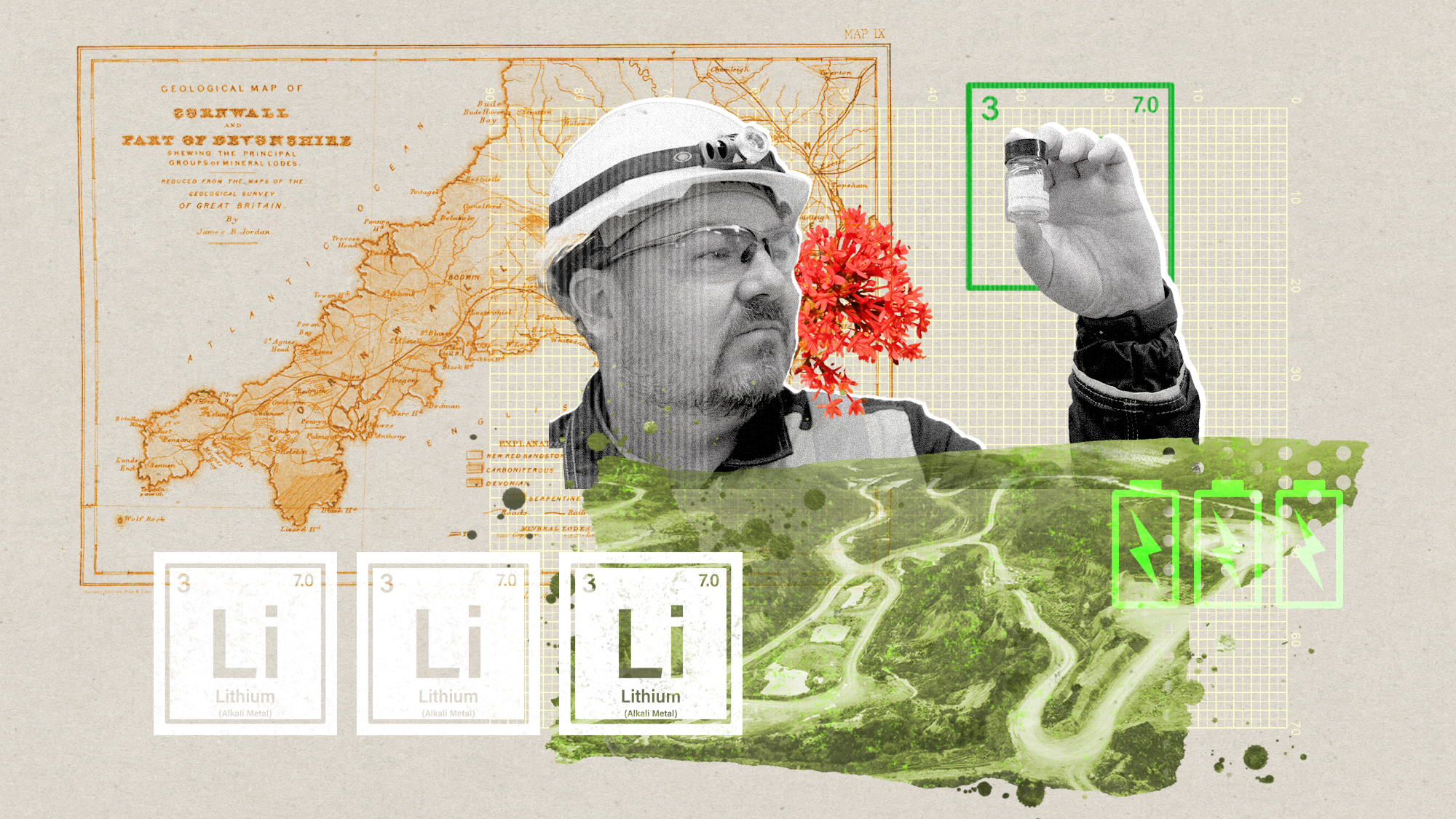 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
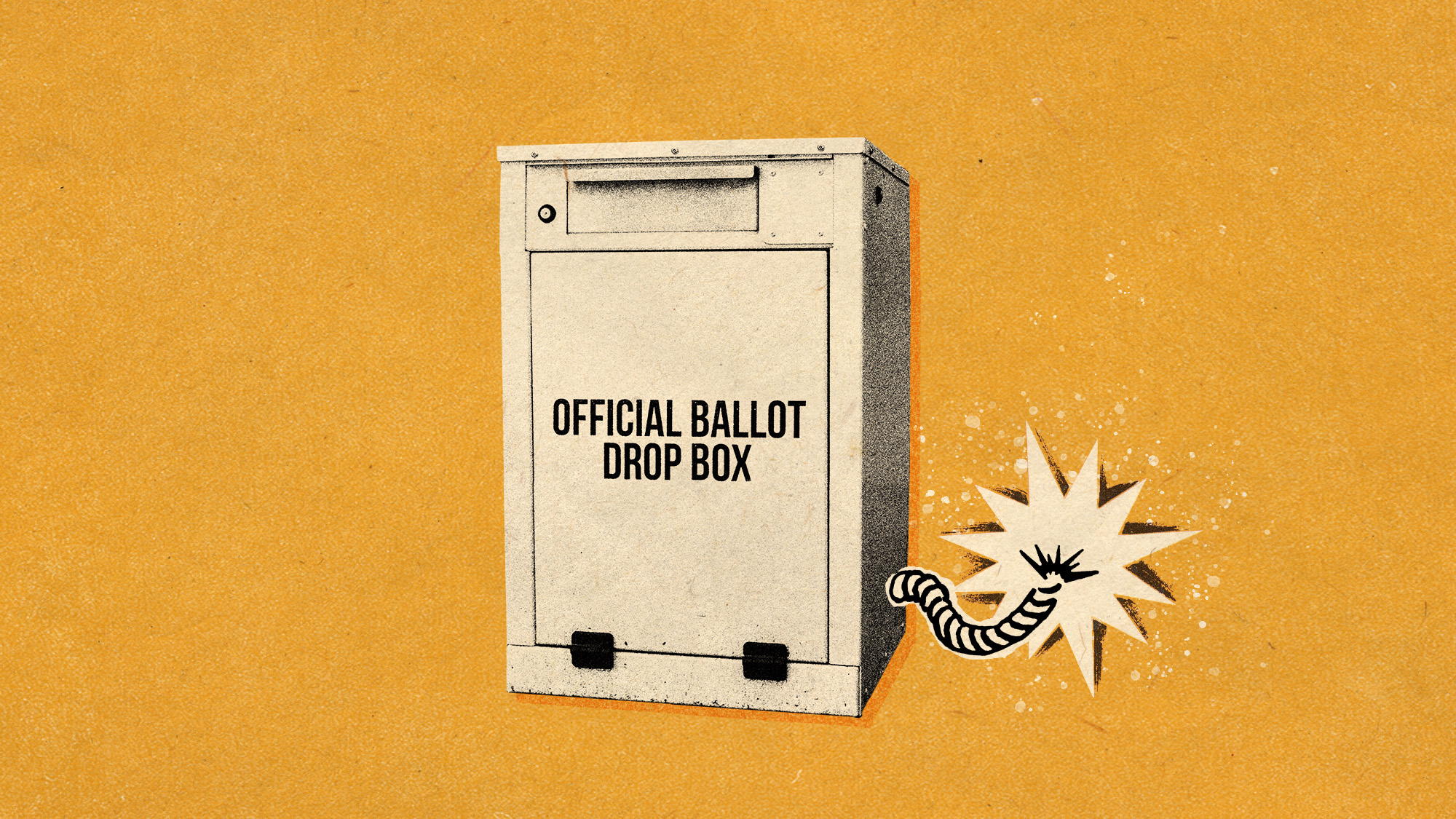 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
