Moderna says COVID-19 vaccine could be approved in December

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Stéphane Bancel, chief executive of Moderna Inc., said Monday that the federal government could grant emergency use authorization for its COVID-19 vaccine in December, assuming the company gets promising data from its Phase 3 trial in November. Moderna started its final-phase trial with 30,000 volunteers in July, and in order to apply for emergency authorization, 53 of the subjects have to become infected with the new coronavirus, and the cases have to be significantly higher in the half of the trial that received a placebo.
That first analysis will likely happen in November, Bancel said at a Wall Street Journal conference, but "it's hard to predict exactly which week because it depends on the cases, the number of people getting sick." If the results don't show sufficient efficacy, Moderna will try again when 106 trial participants contract symptomatic COVID-19, likely delaying authorization until early next year. Under Food and Drug Administration guidelines, drugmakers also have to show that their vaccine is safe for at least two months after vaccination, a benchmark Bancel said Moderna should hit in late November.
Moderna is testing one of four viable COVID-19 vaccines, and its timeline is closest to Pfizer, which said last week it expects to seek emergency use authorization of its vaccine in late November. The trials of vaccine candidates from Johnson & Johnson and AstraZeneca are on pause while the companies look into unexplained health issues among participants. Moderna says it plans to produce 20 million does of its vaccine this year and 500 million next year.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
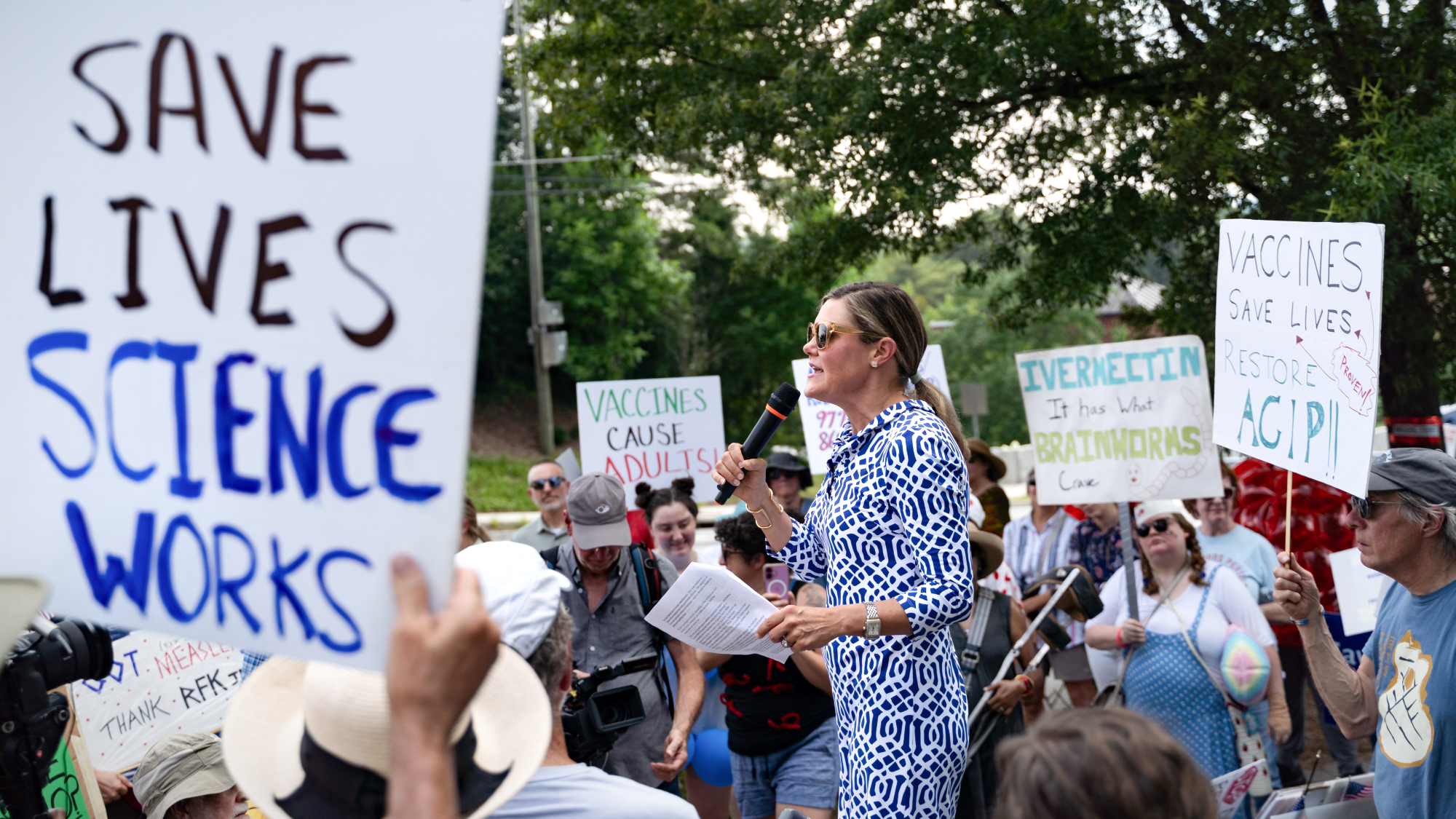 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
