COVID-19 drug trials have struggled to recruit volunteers throughout pandemic

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
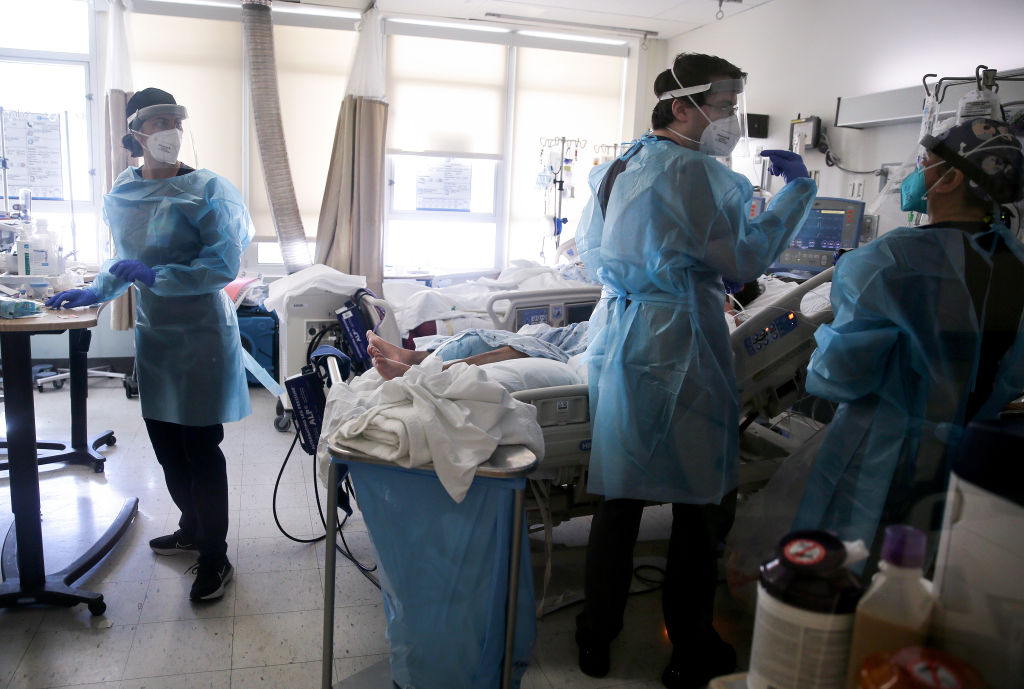
COVID-19 vaccines have been developed at a rapid pace that has largely exceeded expectations, and while there are varying levels of success, several appear to be quite effective. The United States government poured a lot of money into the research, which helped push the development finish line up, but the heavy focus on vaccines did leave coronavirus therapeutics behind, The New York Times reports.
A few drug treatments have helped doctors improve the care of COVID-19 patients, but several ideas that looked like they were gaining steam petered out. One of the key factors was a lack of centralized coordination, the Times suggests. Hospitals and researchers have often been left on their own to conduct trials and many have subsequently struggled to find volunteers who were not already hospitalized.
For instance, the University of Kentucky began a trial in May to test a drug called camostat. Normally used to treat inflammation of the pancreas, camostat's ability to destroy a protein the virus depends on to infect human cells had scientists optimistic it could work as a COVID-19 antiviral. But over the past eight months, the research team just hasn't been able to consistently track down patients who recently received a COVID-19 diagnosis.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"This has been the source of delays for essentially all of the trials around the world," Dr. James Porterfield, an infectious disease clinician at the University of Kentucky College of Medicine, told the Times. Read more at The New York Times.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
