FDA confirms Johnson & Johnson vaccine prevented all deaths and hospitalizations in trial

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The United States could be days away from getting a third COVID-19 vaccine.
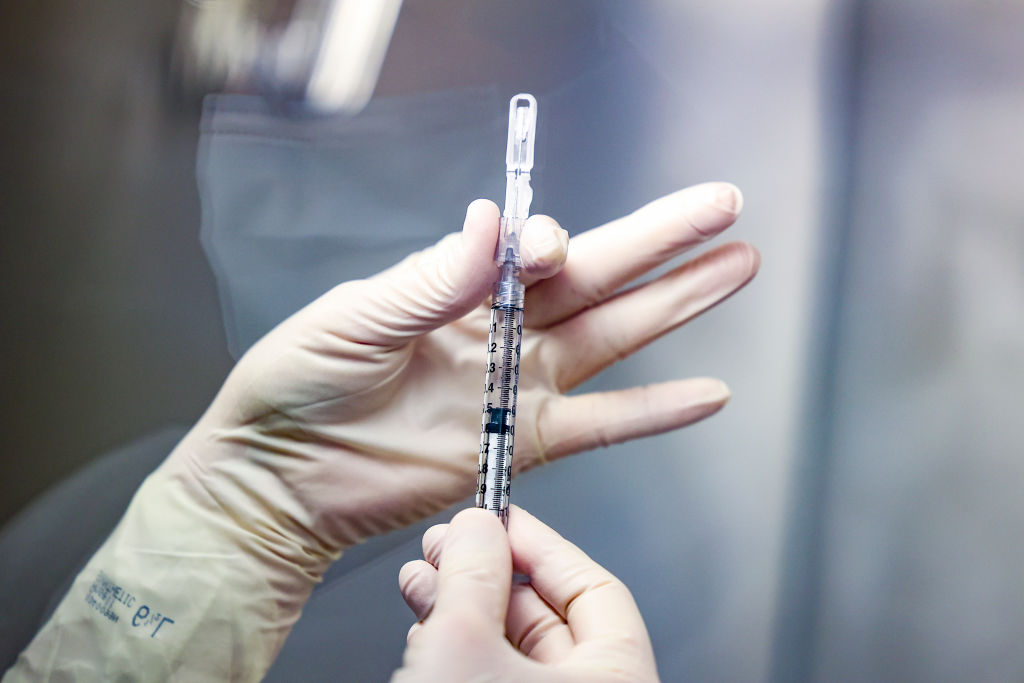
A Food and Drug Administration review has confirmed that Johnson & Johnson's COVID-19 vaccine, which only requires a single shot, is safe and effective, meaning it could be authorized for emergency approval "as soon as this weekend," The Washington Post reports.
The FDA review showed the vaccine was 66 percent effective at preventing moderate to severe COVID-19 in a large clinical trial, though it was 85 percent effective at preventing severe illness. As Johnson & Johnson previously announced, the vaccine also "demonstrated complete protection against COVID-related hospitalization and death, 28 days post-vaccination." That, University of Rochester School of Medicine and Dentistry's Nancy M. Bennett told the Post, is "really what's important."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The Johnson & Johnson vaccine comes with the key benefit of only requiring one dose, as opposed to two doses for both the Pfizer and Moderna vaccines that were previously authorized for emergency approval in the United States. Plus, the Johnson & Johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, The Associated Press notes.
A committee is set to meet Friday to consider whether the FDA should authorize the Johnson & Johnson vaccine for emergency approval. The company says that should the FDA do so, it expects to begin shipping the vaccine immediately and "deliver enough single-doses by the end of March to enable the vaccination of more than 20 million Americans."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
