Coronavirus: how will the Oxford vaccine win regulators’ approval?
A total of three Covid-19 jabs have been declared safe and effective - but none are cleared yet for public use
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
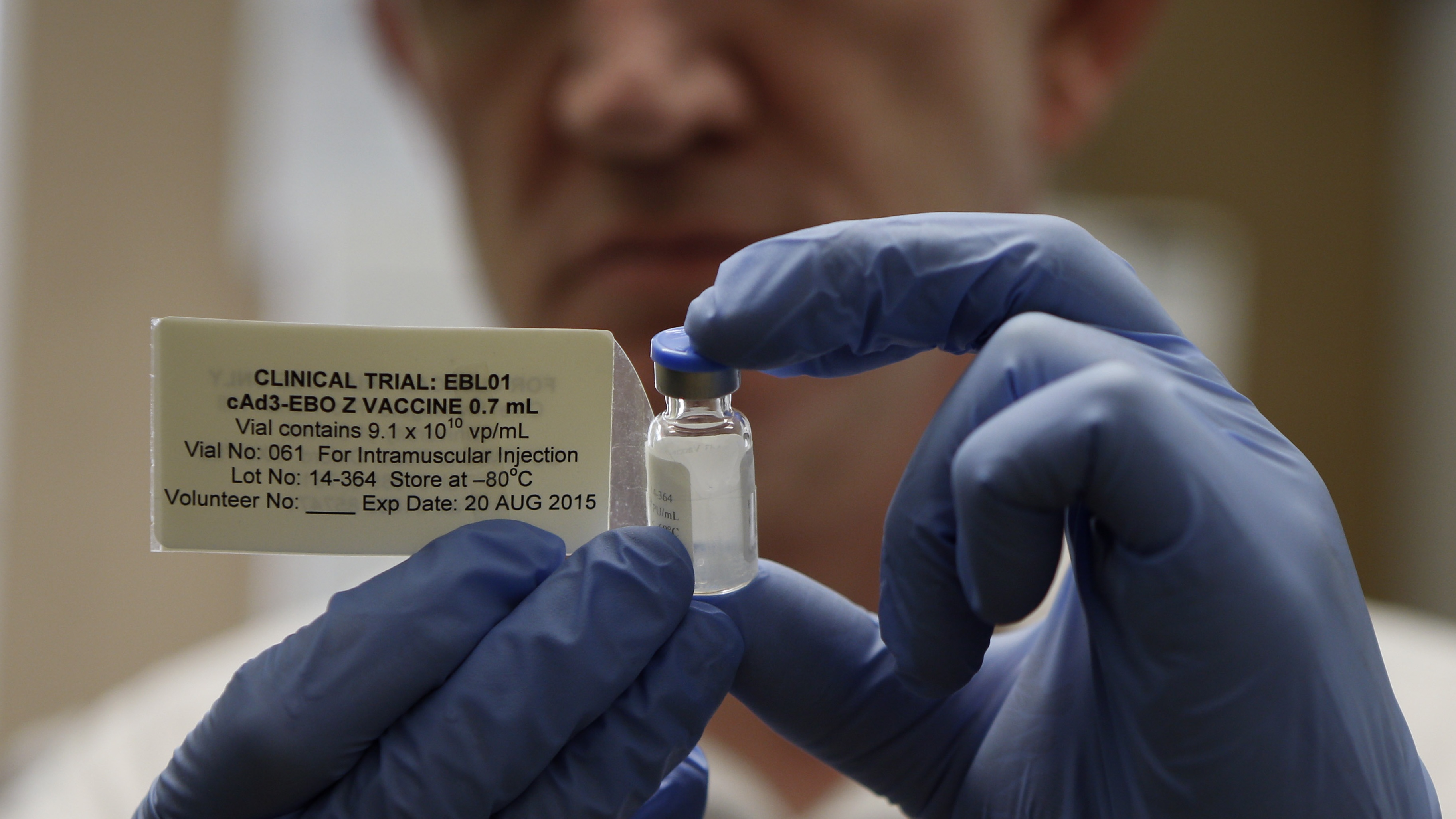
The results of late-stage trials that show the Covid-19 vaccine developed by Oxford University and AstraZeneca is at least 70% effective mark a big step forward - but not the last before the jab can be approved by UK health regulators.
First, the full data from Phase 3 will have to be published in a peer-reviewed journal, supplementing the summary announced earlier this week.
As with preliminary results from the Moderna and Pfizer vaccines, the initial statement “gave efficacy rates [but] left out details that would have helped outside researchers independently assess the data”, says The New York Times.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Medical regulators will want to know exactly how many coronavirus infections - and how many serious infections - were reported in each trial group, including those given a placebo, in order to calculate each vaccine’s efficacy rate for themselves.
This should not take long. Even before the recent trial results announcements, the drug companies had been passing their data to health regulators for “rolling review” in an attempt to accelerate the process.
Dr June Raine, chief executive of the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), has said that her organisation will “aim to make a decision in the shortest time possible, without compromising the thoroughness of our review”.
However, the process won’t end when approval comes, which is likely to be next month. “The MHRA is also involved in pharmacovigilance - monitoring the safety of all medicines throughout their marketed life,” says The Guardian.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
That involves looking out for adverse reactions that may be too rare to show up in trial groups of 20,000 to 30,000 people, but that may affect a significant number of people when hundreds of millions have received the vaccine.
This risk could be reduced by extending the trial period - the meningitis B vaccine took 20 years to gain approval - but the potential dangers of vaccinating has to be weighed against those of not vaccinating.
The “dilemma” is unavoidable, says Nature. Even the impulse to protect trial participants given the placebo comes with drawbacks.
“If too many people cross over to the vaccine group, the companies might not have enough data to establish long-term outcomes, such as safety, how long vaccine protection lasts and whether the jab prevents infection or just the disease,” the journal explains.
Holden Frith is The Week’s digital director. He also makes regular appearances on “The Week Unwrapped”, speaking about subjects as diverse as vaccine development and bionic bomb-sniffing locusts. He joined The Week in 2013, spending five years editing the magazine’s website. Before that, he was deputy digital editor at The Sunday Times. He has also been TheTimes.co.uk’s technology editor and the launch editor of Wired magazine’s UK website. Holden has worked in journalism for nearly two decades, having started his professional career while completing an English literature degree at Cambridge University. He followed that with a master’s degree in journalism from Northwestern University in Chicago. A keen photographer, he also writes travel features whenever he gets the chance.