FDA panel votes unanimously to make birth control pill available over the counter


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. Food and Drug Administration's advisory panel voted unanimously to make the birth control pill Opill available over the counter, saying that the benefits outweigh the risks, The New York Times reports. "I think Opill has the potential to have a huge positive public health impact," said advisory committee member Kathryn Curtis.
While Opill manufacturer Perrigo called the outcome "groundbreaking," this doesn't mean the pill will be available just yet, CNN writes. The FDA is not required to listen to the advisory committee and will decide whether to make the pill available sometime in the summer. Some agency members are concerned about whether people will use the product as directed, especially those with health conditions and adolescents. "The FDA has been put in a very difficult position of trying to determine whether it is likely that women will use this product safely and effectively at the nonprescription setting," explained Dr. Karen Murry, deputy director of the FDA's office of nonprescription drugs.
The push to approve Opill is stronger than ever as many states have adopted extra-restrictive abortion bans following the overturning of Roe v. Wade (1973). Maternal healthcare has also reportedly been on a decline because of these laws. "I think this represents a landmark in our history of women's health," commented advisor Dr. Marjorie Gass said of the FDA progress. "Unwanted pregnancies can really derail a woman's life, and especially an adolescent's life."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"We are motivated by the millions of people who need easy access to safe and effective contraception," remarked Perrigo president and CEO Murray S. Kessler in a statement. "Today's outcome reflects Perrigo's steadfast commitment to women and people, and their health."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Devika Rao has worked as a staff writer at The Week since 2022, covering science, the environment, climate and business. She previously worked as a policy associate for a nonprofit organization advocating for environmental action from a business perspective.
-
 The Week Unwrapped: Do the Freemasons have too much sway in the police force?
The Week Unwrapped: Do the Freemasons have too much sway in the police force?Podcast Plus, what does the growing popularity of prediction markets mean for the future? And why are UK film and TV workers struggling?
-
 Properties of the week: pretty thatched cottages
Properties of the week: pretty thatched cottagesThe Week Recommends Featuring homes in West Sussex, Dorset and Suffolk
-
 The week’s best photos
The week’s best photosIn Pictures An explosive meal, a carnival of joy, and more
-
 Scientists are worried about amoebas
Scientists are worried about amoebasUnder the radar Small and very mighty
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Deaths of children under 5 have gone up for the first time this century
Deaths of children under 5 have gone up for the first time this centuryUnder the radar Poor funding is the culprit
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 Stopping GLP-1s raises complicated questions for pregnancy
Stopping GLP-1s raises complicated questions for pregnancyThe Explainer Stopping the medication could be risky during pregnancy, but there is more to the story to be uncovered
-
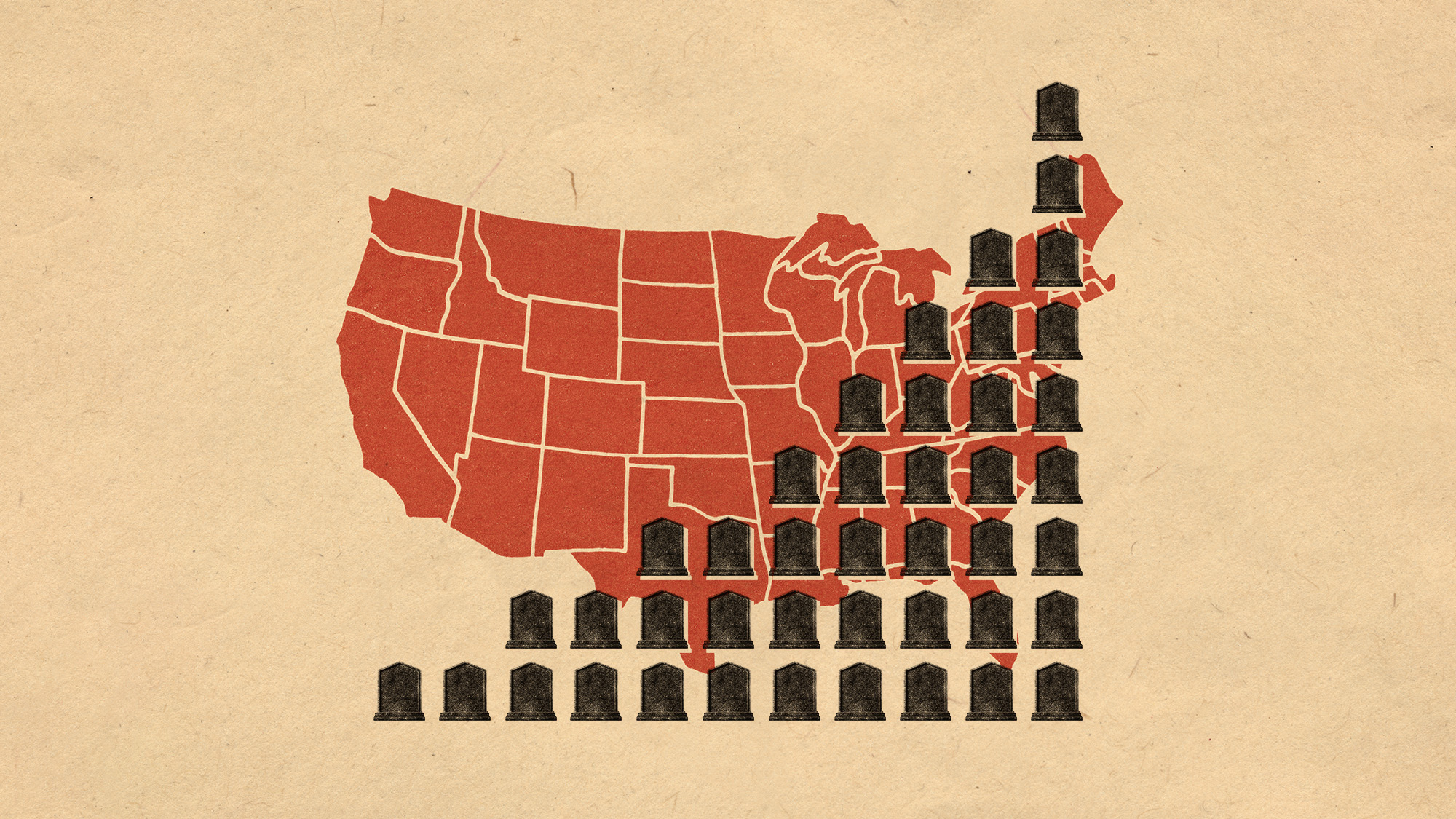 More adults are dying before the age of 65
More adults are dying before the age of 65Under the radar The phenomenon is more pronounced in Black and low-income populations
