Black patients are often left out of crucial cancer drug trials


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
In clinical trials for the majority of FDA-approved cancer drugs, fewer than 5 percent of the patients were black, Stat News and ProPublica reported Wednesday.
Out of the 31 cancer drugs approved since 2015, 24 of them have had single-digit proportions of black patients during trials, the analysis found. In one trial for a multiple myeloma treatment, just 1.8 percent of participants were black, even though black Americans are twice as likely as white Americans to be diagnosed with the blood cancer and there may be "meaningful differences" in how the condition affects the two races.
The Food and Drug Administration has not established any rules that would require drug makers to test treatments on minority patients, and many manufacturers don't diversify their trials voluntarily, reports Stat News.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Drug companies often say it is challenging to enroll minorities, reports ProPublica. The clinical trials with the highest black participation, up to 12 percent, were from Johnson & Johnson, a company that uses an internal group to improve trial diversity. Advocates have called on the FDA to implement similar standards across the industry, but the agency has demurred.
Minorities are also often not properly incentivized or are not able to participate: Financial barriers, logistical challenges, and distrust of the medical community are all factors that sometimes discourage minorities from joining trials, even though they could be "life-extending opportunities," said Dr. Kashif Ali, research head at Maryland Oncology Hematology. Read more at Stat News.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Summer Meza has worked at The Week since 2018, serving as a staff writer, a news writer and currently the deputy editor. As a proud news generalist, she edits everything from political punditry and science news to personal finance advice and film reviews. Summer has previously written for Newsweek and the Seattle Post-Intelligencer, covering national politics, transportation and the cannabis industry.
-
 The 8 best TV shows of the 1960s
The 8 best TV shows of the 1960sThe standout shows of this decade take viewers from outer space to the Wild West
-
 Microdramas are booming
Microdramas are boomingUnder the radar Scroll to watch a whole movie
-
 The Olympic timekeepers keeping the Games on track
The Olympic timekeepers keeping the Games on trackUnder the Radar Swiss watchmaking giant Omega has been at the finish line of every Olympic Games for nearly 100 years
-
 Scientists are worried about amoebas
Scientists are worried about amoebasUnder the radar Small and very mighty
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Deaths of children under 5 have gone up for the first time this century
Deaths of children under 5 have gone up for the first time this centuryUnder the radar Poor funding is the culprit
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
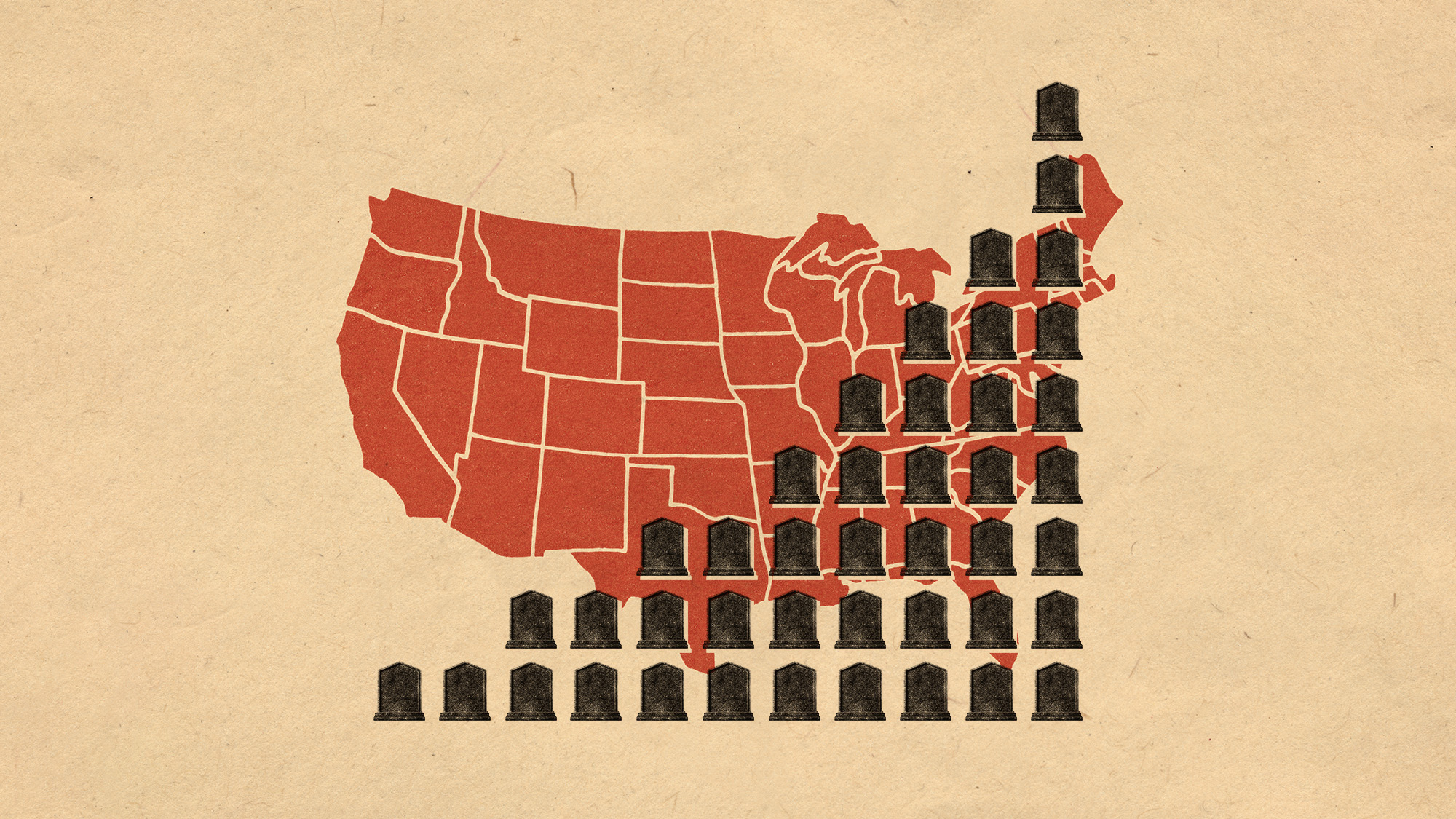 More adults are dying before the age of 65
More adults are dying before the age of 65Under the radar The phenomenon is more pronounced in Black and low-income populations
-
 Ultra-processed America
Ultra-processed AmericaFeature Highly processed foods make up most of our diet. Is that so bad?
