Scientists decry NIH's decision to end remdesivir study as 'a lost opportunity'

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
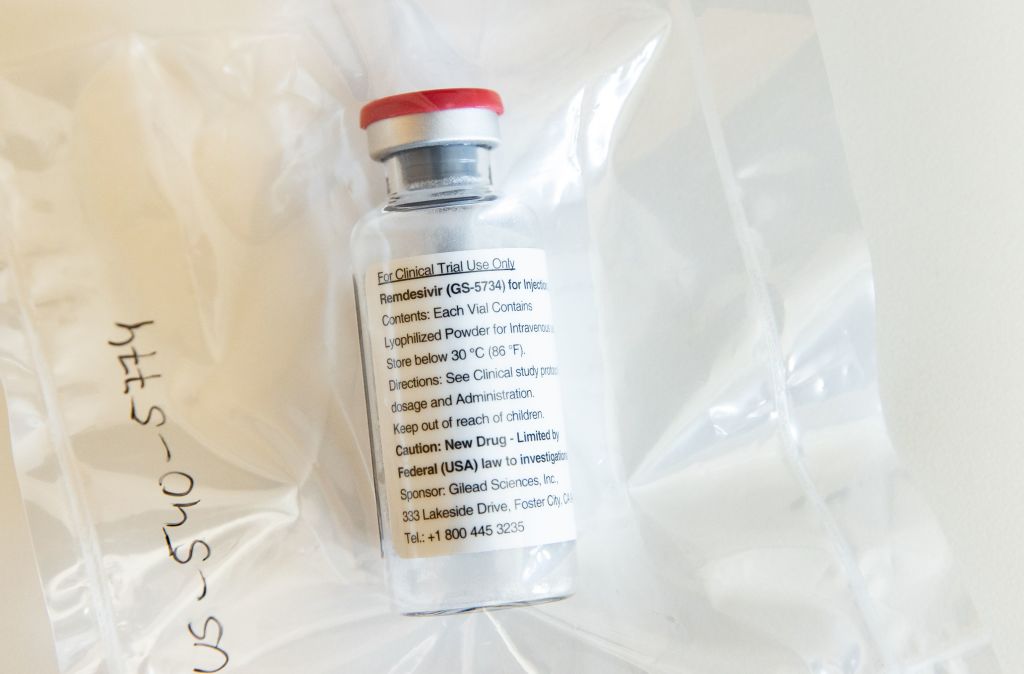
Most scientists agree the National Institute of Allergy and Infectious Diseases, one of the 27 branches that make up the National Institutes of Health, faced a difficult decision when it decided to end a coronavirus treatment study early and begin giving remdesivir to patients assigned to receive a placebo after finding that the antiviral drug reduced recovery time. The call has received a lot of support, especially because it was made during a pandemic, and NIAID, considering it a moral imperative, has no regrets, Stat News reports. But there are some holdouts.
Steven Nissen, a trialist and cardiologist at the Cleveland Clinic, and Peter Bach, the director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, don't think recovery time reduction should have been the deciding factor. Instead, they say, the study should have continued until the NIAID was able to collect more data on mortality — the survival rate for coronavirus patients enrolled in the trial wasn't statistically significant when the study ended. "The question is: Was there a route, or is there a route, to determine if the drug can prevent death?," said Nissen.
Without getting a clearer answer to that question, he said, the study is a "lost opportunity."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The NIAID's clinical director, H. Clifford Lane, has a counter argument. He wants to know how "many patients would we want to put at risk of dying" to finalize a more complete study. Read more at Stat News.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 Regent Hong Kong: a tranquil haven with a prime waterfront spot
Regent Hong Kong: a tranquil haven with a prime waterfront spotThe Week Recommends The trendy hotel recently underwent an extensive two-year revamp
-
 The problem with diagnosing profound autism
The problem with diagnosing profound autismThe Explainer Experts are reconsidering the idea of autism as a spectrum, which could impact diagnoses and policy making for the condition
-
 Blue Origin launches Mars probes in NASA debut
Blue Origin launches Mars probes in NASA debutSpeed Read The New Glenn rocket is carrying small twin spacecraft toward Mars as part of NASA’s Escapade mission
-
 Dinosaurs were thriving before asteroid, study finds
Dinosaurs were thriving before asteroid, study findsSpeed Read The dinosaurs would not have gone extinct if not for the asteroid
-
 SpaceX breaks Starship losing streak in 10th test
SpaceX breaks Starship losing streak in 10th testspeed read The Starship rocket's test flight was largely successful, deploying eight dummy satellites during its hour in space
-
 Rabbits with 'horns' sighted across Colorado
Rabbits with 'horns' sighted across Coloradospeed read These creatures are infected with the 'mostly harmless' Shope papilloma virus
-
 Lithium shows promise in Alzheimer's study
Lithium shows promise in Alzheimer's studySpeed Read Potential new treatments could use small amounts of the common metal
-
 Scientists discover cause of massive sea star die-off
Scientists discover cause of massive sea star die-offSpeed Read A bacteria related to cholera has been found responsible for the deaths of more than 5 billion sea stars
-
 'Thriving' ecosystem found 30,000 feet undersea
'Thriving' ecosystem found 30,000 feet underseaSpeed Read Researchers discovered communities of creatures living in frigid, pitch-black waters under high pressure
-
 New York plans first nuclear plant in 36 years
New York plans first nuclear plant in 36 yearsSpeed Read The plant, to be constructed somewhere in upstate New York, will produce enough energy to power a million homes
