Human trials of Oxford coronavirus vaccine paused over ‘spinal-cord disease fears’
Leaked report says volunteer in UK was left unable to walk after receiving two doses
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
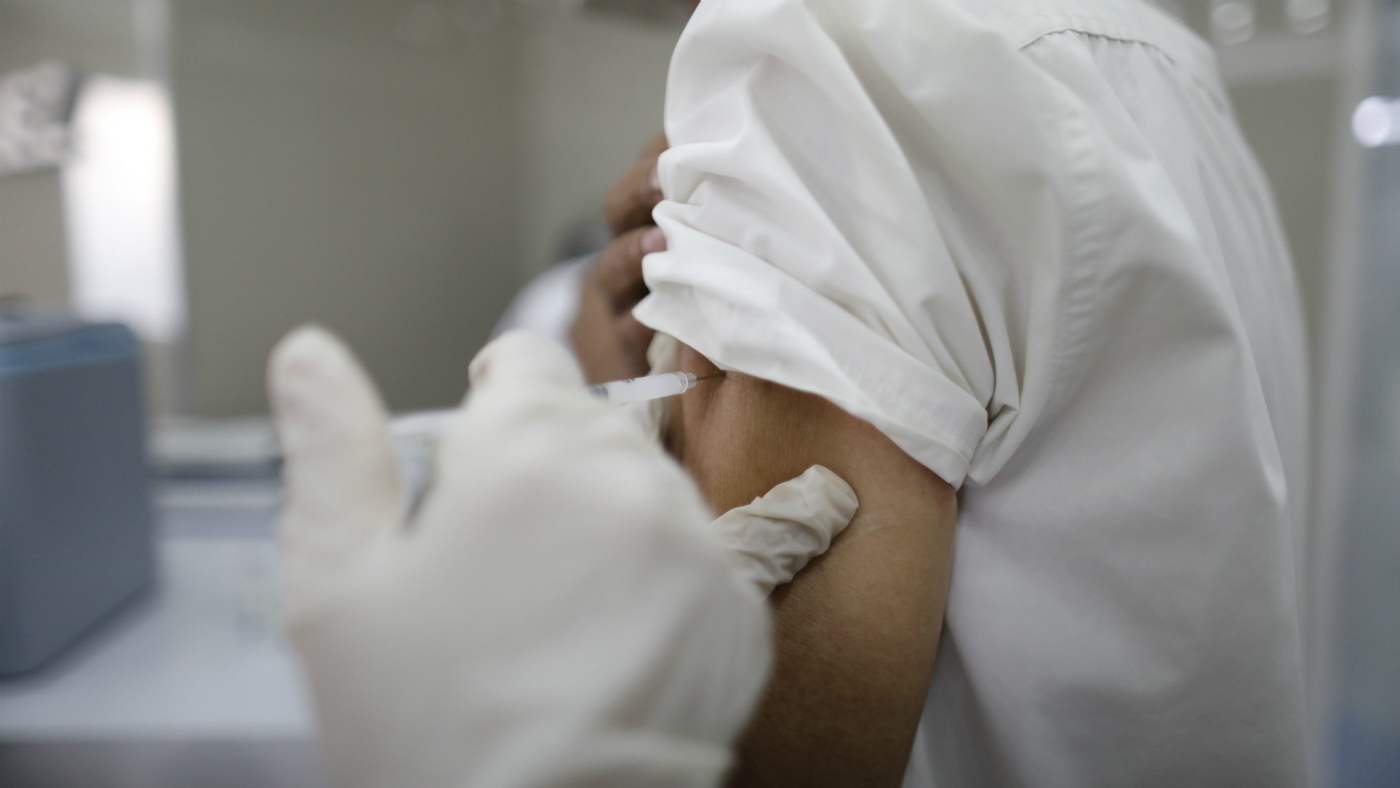
Human trials of the Oxford coronavirus vaccine are facing delays in the US after a participant suffered a rare neurological condition, according to a leaked document.
The AstraZeneca report, seen by CNN, shows that the decision to pause the trials in early September was made after a previously healthy 37-year-old woman who had received two doses developed transverse myelitis, an inflammation of the spinal cord that can cause paralysis.
The unidentified UK-based volunteer was given the jabs in June and August but was hospitalised after she started to suffer “difficulty walking, pain and weakness in her arms and a headache after tripping while running in September”, the Daily Mail reports.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
She is understood to have recovered fully.
The internal report by AstraZeneca, which owns the rights to the so-called AZD1222 vaccine, also reveals that the trials had been stopped before after another participant developed transverse myelitis.
“The first pause, in July, was not publicly revealed and the trial was restarted after it was determined the volunteer had multiple sclerosis, a condition that can cause the same neurological reaction,” says The Telegraph.
Following the latest hold-up, trials have resumed in the UK, Brazil, India and South Africa, but remain suspended in the US.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The “concerns around neurological side-effects” of vaccines are “especially sensitive in the US”, according to the newspaper, which notes that an emergency influenza vaccine issued in 1976 caused 450 cases, of which 30 were fatal, of Guillain-Barre syndrome, which also causes inflammation of the spinal cord.
The US Food and Drug Administration (FDA) and the National Institutes of Health (NIH) are reportedly “seeking to determine” what caused the case of transverse myelitis in the Covid trials before giving the green light for the testing to resume.
-
 Secured vs. unsecured loans: how do they differ and which is better?
Secured vs. unsecured loans: how do they differ and which is better?the explainer They are distinguished by the level of risk and the inclusion of collateral
-
 ‘States that set ambitious climate targets are already feeling the tension’
‘States that set ambitious climate targets are already feeling the tension’Instant Opinion Opinion, comment and editorials of the day
-
 Mixing up mixology: The year ahead in cocktail and bar trends
Mixing up mixology: The year ahead in cocktail and bar trendsthe week recommends It’s hojicha vs. matcha, plus a whole lot more
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
-
 Vaccine critic quietly named CDC’s No. 2 official
Vaccine critic quietly named CDC’s No. 2 officialSpeed Read Dr. Ralph Abraham joins another prominent vaccine critic, HHS Secretary Robert F. Kennedy Jr.
-
 This flu season could be worse than usual
This flu season could be worse than usualIn the spotlight A new subvariant is infecting several countries
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system