The FDA's review of the Moderna vaccine for teens could take until January 2022

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
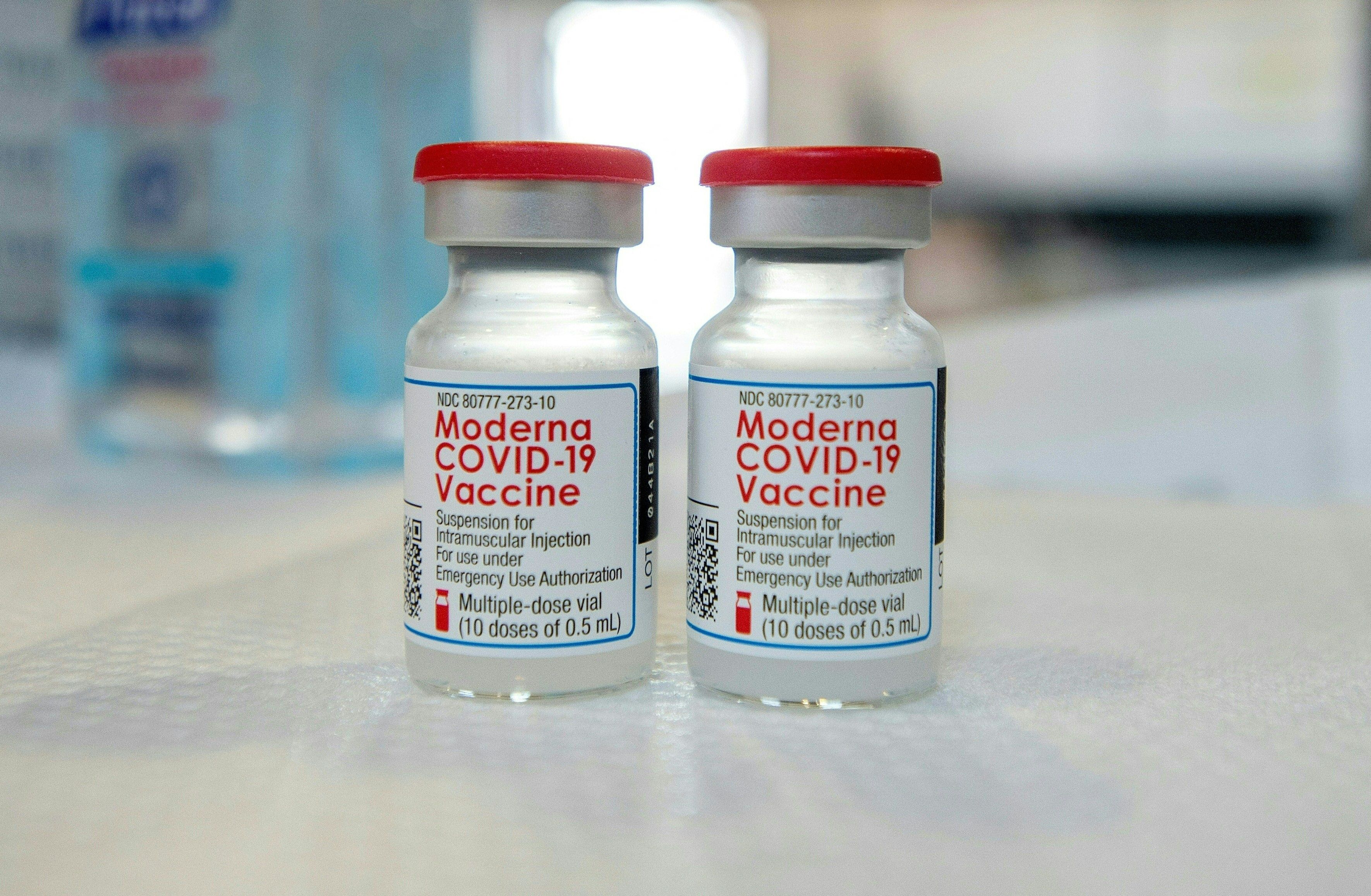
The Food and Drug Administration needs more time to complete its assessment of Moderna's COVID-19 vaccine for use for 12- to 17-year-olds, the drugmaker said Sunday. The review could take until January 2022 to complete.
Moderna said in May that its vaccine was 100 percent effective in a study of 12- to 17-year-olds. The FDA is now specifically reviewing the risk of myocarditis, an inflammation of the heart muscle. Moderna will wait to file a request for emergency authorization for a smaller dose of its vaccine for children ages 6 to 11 until the FDA completes the review for the teenage group.
The FDA expanded Pfizer's COVID-19 emergency use to include adolescents ages 12 through 15 back in May. On Friday, the FDA further approved Pfizer's COVID-19 vaccine for emergency use authorization for children ages 5 to 11.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Jeva Lange was the executive editor at TheWeek.com. She formerly served as The Week's deputy editor and culture critic. She is also a contributor to Screen Slate, and her writing has appeared in The New York Daily News, The Awl, Vice, and Gothamist, among other publications. Jeva lives in New York City. Follow her on Twitter.
-
 How to Get to Heaven from Belfast: a ‘highly entertaining ride’
How to Get to Heaven from Belfast: a ‘highly entertaining ride’The Week Recommends Mystery-comedy from the creator of Derry Girls should be ‘your new binge-watch’
-
 The 8 best TV shows of the 1960s
The 8 best TV shows of the 1960sThe standout shows of this decade take viewers from outer space to the Wild West
-
 Microdramas are booming
Microdramas are boomingUnder the radar Scroll to watch a whole movie
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
