Brazil expands approval of chloroquine to treat COVID-19. Utah canceled its big bet on the unproven drug.

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
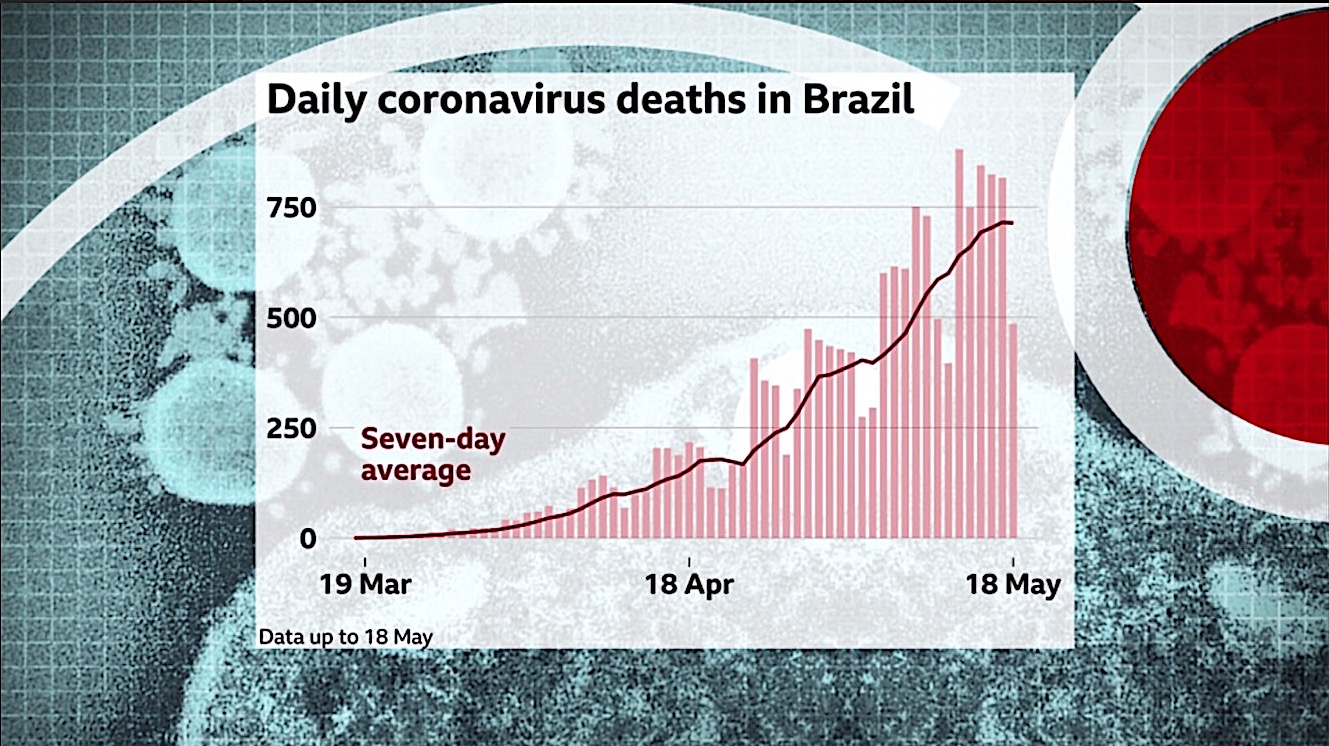
Brazilian President Jair Bolsonaro announced Wednesday that the Health Ministry had expanded approved uses of the anti-malaria drug chloroquine to treat milder cases of COVID-19. Brazil now has the world's third-largest coronavirus recorded outbreak, after the U.S. and Russia, and hospitals and health systems in several states have gone over capacity. "There is still no scientific evidence, but it is being monitored and used in Brazil and worldwide," Bolsonaro said on his Facebook page.
Chloroquine is the predecessor of hydroxychloroquine, which President Trump says he is now taking in a preventative capacity. Bolsonaro, a conservative populist and admirer of Trump, has continually downplayed the severity of COVID-19 and is clashing with cities and states that have enacted lockdowns and other coronavirus mitigation efforts. Brazil ended one study of chloroquine after detecting an increase in heart arrhythmia, and several large observational studies have found no positive effect of the drug on COVID-19 patients and some negative outcomes.
The order to expand approved use of chloroquine was signed by interim Health Minister Gen. Eduardo Pazuello, who had no health experience when Bolsonaro made him the No. 2 official at the ministry in April. His appointment followed Bolsonaro's firing of Health Minister Luiz Henrique Mandetta after he publicly supported governors pursuing mitigation measures, and Pazuello got the top job when Mandetta's successor, Nelson Teich, resigned last week after he publicly clashed with Bolsonaro over chloroquine.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.

Utah, meanwhile, caught hydroxychlorquine fever early, preparing plans to allow the drug to be distributed without prescription and ordering at least $800,000 worth of chloroquine and hydroxychloroquine from a pharmacist who was helping draw up those plans, Stat News reported Sunday. Utah canceled the order in late April and got a refund.
Hydroxychloroquine's rise and fall in Utah "provides a case study of what happens when hope and excitement about therapies outpace the evidence," Stat reports. "It underscores the pressure officials felt to demonstrate they were on top of the response, even as such efforts sowed confusion among the medical community and led them into initiatives they came to regret. And, mirroring the hydroxychloroquine debate in the Trump administration, it shows how experts scrambled to inject restraint and plead for leaders to follow evidence at a time when promises of easy remedies were more enticing." Read more about Utah's experience at Stat News.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 ‘This is something that happens all too often’
‘This is something that happens all too often’Instant Opinion Opinion, comment and editorials of the day
-
 House votes to end Trump’s Canada tariffs
House votes to end Trump’s Canada tariffsSpeed Read Six Republicans joined with Democrats to repeal the president’s tariffs
-
 Bondi, Democrats clash over Epstein in hearing
Bondi, Democrats clash over Epstein in hearingSpeed Read Attorney General Pam Bondi ignored survivors of convicted sex offender Jeffrey Epstein and demanded that Democrats apologize to Trump
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
